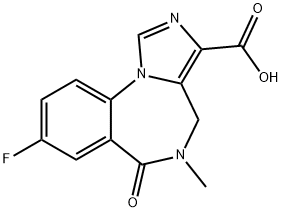
4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO- synthesis
- Product Name:4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-
- CAS Number:84378-44-9
- Molecular formula:C13H10FN3O3
- Molecular Weight:275.24
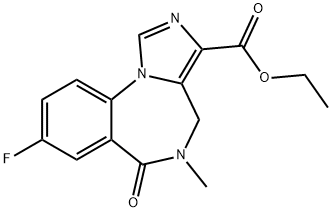
78755-81-4
![4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-](/CAS/GIF/84378-44-9.gif)
84378-44-9
The general procedure for the synthesis of 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[F]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid from ethyl 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate is as follows: 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[1,5-a][1,4]diazepine-3-carboxylic acid was prepared from 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[1,5-a][1,4]diazepine-3-carboxylic acid as follows. -6-oxo-5,6-dihydro-4H-benzo[F]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid ethyl ester (0.5 g, 1.7 mmol) was suspended in tetrahydrofuran (THF, 50 mL). Aqueous sodium hydroxide (2M, 10 mL) was added to the suspension to completely dissolve the solid. The reaction mixture was stirred at room temperature until completion of the reaction was confirmed by thin layer chromatography (TLC) monitoring. Upon completion of the reaction, the solvent was evaporated under reduced pressure. The residue was dissolved in water and acidified with 2M hydrochloric acid to precipitate the target product as a solid. The solid product was collected by filtration and dried under vacuum to afford 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid as a colorless solid (0.45 g, 100% yield). The structure of the product was confirmed by 1H NMR (CD3OD): δ 3.24 (3H, s, CH3), 4.62 (1H, br d, J = 15.4 Hz, CHaHb), 5.19 (1H, br d, J = 15.4 Hz, CHaHb), 7.62 (1H, ddd, JHF = 12.5 Hz, JHH = 7.7 and 3.1 Hz. CFCHCH), 7.79 (1H, dd, JHF = 8.9 Hz, JHH = 3.1 Hz, CCHCF), 7.87 (1H, dd, JHF = 9.1 Hz, JHH = 4.5 Hz, CHCHCF), 9.63 (1H, s, NCHN).

78755-81-4
383 suppliers
$17.00/5mg
![4H-IMIDAZO[1,5-A][1,4]BENZODIAZEPINE-3-CARBOXYLIC ACID, 8-FLUORO-5,6-DIHYDRO-5-METHYL-6-OXO-](/CAS/GIF/84378-44-9.gif)
84378-44-9
83 suppliers
$80.00/5mg
Yield: 100%
Reaction Conditions:
Stage #1:flumazenil with sodium hydroxide in tetrahydrofuran;water at 20;
Stage #2: with hydrogenchloride in water
Steps:
3.1: 8-Fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid
Flumazenil (0.5 g, 1.7 mmol) was suspended in THF (50 mL). Sodium hydroxide (10 mL of a 2M solution in water) was added and the suspended solid dissolved. The mixture was stirred at room temperature until judged complete by thin layer chromatography (TLC). The solvent was removed in vacuo and the residue dissolved in water, treated with 2M hydrochloric acid to precipitate the acid. The solid was filtered off and dried in vacuo to give a colourless solid (0.45 g, 100%). 1H NMR (CD3OD): dH 3.24 (3H. s, CH3), 4.62 (1H, br d, J = 15.4 Hz, CHaHb), 5.19 (1H, br d, J = 15.4 Hz, CHaHb), 7.62 (1H, ddd, JHF = 12.5 Hz, JHH = 7.7 and 3.1 Hz, CFCHCH), 7.79 (1H, dd, JHF = 8.9 Hz, JHH = 3.1 Hz, CCHCF), 7.87 (1H, dd, JHF = 9.1 Hz, JHH = 4.5 Hz, CHCHCF), and 9.63 (1H, s, NCHN).
References:
Jackson, Alexander;Guilbert, Benedicte B.;Plant, Stuart D.;Goggi, Julian;Battle, Mark R.;Woodcraft, John L.;Gaeta, Alessandra;Jones, Clare L.;Bouvet, Denis R.;Jones, Paul A.;O'Shea, Dennis M.;Zheng, Penny Hao;Brown, Samantha L.;Ewan, Amanda L.;Trigg, William [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 3,p. 821 - 826] Location in patent:supporting information