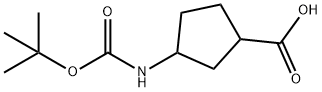
3-((tert-Butoxycarbonyl)aMino)cyclopentanecarboxylic acid synthesis
- Product Name:3-((tert-Butoxycarbonyl)aMino)cyclopentanecarboxylic acid
- CAS Number:855863-93-3
- Molecular formula:C11H19NO4
- Molecular Weight:229.27

24424-99-5
861 suppliers
$13.50/25G
89614-96-0
81 suppliers
$44.79/100MG

855863-93-3
44 suppliers
$47.00/250mg
Yield:-
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;water for 16 h;
Steps:
13.13a 13a) 3-(3-tert-Butoxycarbonylamino-cyclopentyl)-3-oxo-propionic acid methyl ester
13a) 3-(3-tert-Butoxycarbonylamino-cyclopentyl)-3-oxo-propionic acid methyl ester A stirred mixture of 3-aminocyclopentanecarboxylic acid (4.00 g, 31 .0 mmol) in THF (20 ml_) was treated with NaOH (1 M aqueous, 57 ml_) followed by Boc anhydride (6.76 g, 31 .0 mmol). After 16 h the mixture was acidified with 2N HCI and then extracted with EtOAc (2x 70 ml_). The organic phases were combined, dried (MgS04), filtered, and concentrated to dryness. The residue was taken up into dry THF (70 ml_) and 1 ,1 '- carbonyldiimidazole (2.86 g, 17.6 mmol) was added and the mixture stirred for 1 h. A solid, powdered mixture of MgCI2 (2.10 g, 17.6 mmol) and potassium methyl malonate (5.05 g, 32.3 mmol) was added and the mixture stirred at room temperature for 18h. The mixture was concentrated and then partitioned between EtOAc and water. The aqueous phase was extracted with EtOAc before the combined organic phase was washed with brine, dried (MgS04), filtered, and concentrated to dryness to give the product (5g, quantative) used directly in the next step without purification.
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;ASTEX THERAPEUTICS LIMITED;CALLAHAN, James Francis;KERNS, Jeffrey K.;LI, Peng;LI, Tindy;MCCLELAND, Brent W.;NIE, Hong;PERO, Joseph E.;DAVIES, Thomas Glanmor;GRAZIA CARR, Maria;GRIFFITHS-JONES, Charlotte Mary;HEIGHTMAN, Thomas Daniel;NORTON, David;VERDONK, Marinus Leendert;WOOLFORD, Alison Jo-Anne;WILLEMS, Hendrika Maria Gerarda WO2017/60854, 2017, A1 Location in patent:Page/Page column 180