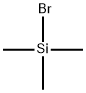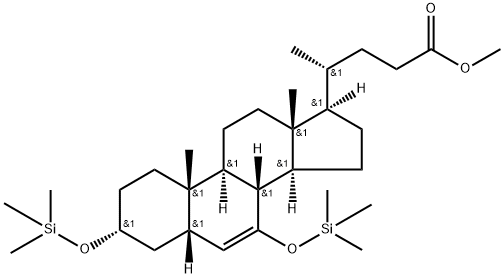
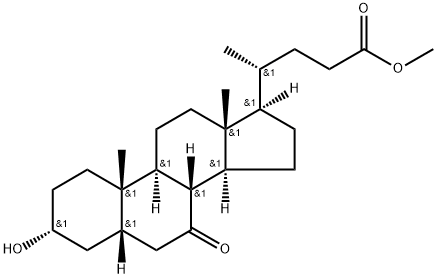
(3α,5β)-3,7-bis[(trimethylsilyl)oxy]-chol-6-en-4-oic acid methyl ester (BTC-D1) synthesis
- Product Name:(3α,5β)-3,7-bis[(trimethylsilyl)oxy]-chol-6-en-4-oic acid methyl ester (BTC-D1)
- CAS Number:863239-58-1
- Molecular formula:C31H56O4Si2
- Molecular Weight:548.96
Yield:863239-58-1 100%
Reaction Conditions:
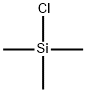
Stage #1: chloro-trimethyl-silanewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane; for 0.166667 h;Inert atmosphere;Cooling with acetone-dry ice;
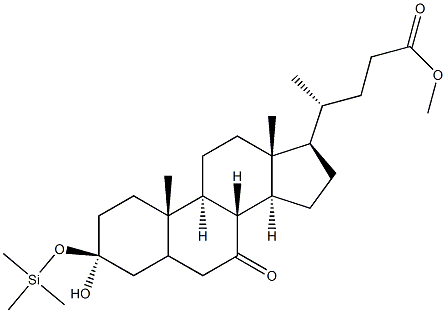
Stage #2: 7-ketolithocholic methyl ester in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Steps:
1.3 Synthesis of Compound 1.3
In a 100 mL three-necked flask under nitrogen protectionAnhydrous tetrahydrofuran (10 mL) and diisopropylamine (1.5 g, 14.9 mmol)And in dry ice acetone bath,The inside temperature was cooled to -78 ° C.Under nitrogen protection,A mixture of n-butyllithium (6 mL, 15 mmol,2.5M n-hexane solution) was slowly added dropwise,And stirred at -78 & lt; 0 & gt; C for 0.5 h.Under nitrogen protection,Trimethylchlorosilane (TMSCl) (2.15 g, 19.9 mmol) was slowly added dropwise,After stirring for 10 minutes,Compound 1.2 (1 g, 2.5 mmol)Was dissolved in anhydrous tetrahydrofuran (5 mL)And under nitrogen protection,Slowly dripping into the reaction system,Stirring at -78 ° C for 0.5 hour,Under a nitrogen atmosphere, triethylamine (3.75 g, 37 mmol) was added,The reaction was stirred at -78 ° C for 1 hour,The internal temperature of the reaction solution gradually increased to -20 ° C,A saturated aqueous solution of sodium bicarbonate (10 mL) was added to the reaction solution, and the reaction solution was allowed to stand at room temperature, and the layers were allowed to stand and the aqueous phase was extracted with ethyl acetate (3 x 20 mL). The organic phases were combined, washed with saturated aqueous sodium bicarbonate, water and saturated brine, dried over anhydrous sodium sulfate, and the filtrate was distilled under reduced pressure to give Compound 1.3 (1.4 g, yield: 100%).
References:
CN106518946,2017,A Location in patent:Paragraph 0138; 0139; 0146; 0147
75-77-4
592 suppliers
$5.04/10
77341-09-4
15 suppliers
$452.00/250mg
![(3α,5β)-3,7-bis[(trimethylsilyl)oxy]-chol-6-en-4-oic acid methyl ester (BTC-D1)](/CAS/20211123/GIF/863239-58-1.gif)
863239-58-1
11 suppliers
inquiry
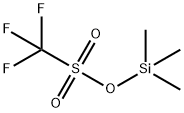
10538-59-7
135 suppliers
inquiry
![(3α,5β)-3,7-bis[(trimethylsilyl)oxy]-chol-6-en-4-oic acid methyl ester (BTC-D1)](/CAS/20211123/GIF/863239-58-1.gif)
863239-58-1
11 suppliers
inquiry
27607-77-8
516 suppliers
$10.00/5g

77341-09-4
15 suppliers
$452.00/250mg
![(3α,5β)-3,7-bis[(trimethylsilyl)oxy]-chol-6-en-4-oic acid methyl ester (BTC-D1)](/CAS/20211123/GIF/863239-58-1.gif)
863239-58-1
11 suppliers
inquiry