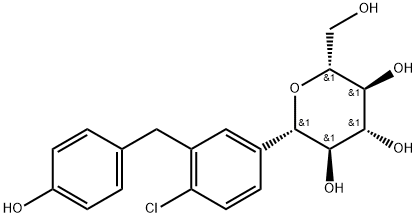
((2R,3S,4R,5R,6S)-6-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)Methyl acetate synthesis
- Product Name:((2R,3S,4R,5R,6S)-6-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)Methyl acetate
- CAS Number:864070-37-1
- Molecular formula:C19H21ClO6
- Molecular Weight:380.82
1079083-63-8
40 suppliers
inquiry

864070-37-1
95 suppliers
inquiry
Yield:864070-37-1 196 g
Reaction Conditions:
with lithium hydroxide monohydrate;water in tetrahydrofuran;methanol at 20 - 25; for 16 h;
Steps:
3 step 3
In a 10L reaction flask, add the crude product obtained in the previous step (theoretical value 0.52mol),Add tetrahydrofuran (1.3L), methanol (2L), water (665mL), stir for 30min,Add lithium hydroxide monohydrate (109.2g, 2.6mol), stir at 2025 for 16h, HPLC monitors the completion of the reaction, the reaction solution is concentrated under reduced pressure to remove part of the solvent (about 1L remaining),Add dilute hydrochloric acid (1mol/L) to the residue to adjust the pH to 6-7, add ethyl acetate to the aqueous phase and extract three times, 1.5L each time, combine the organic phases, and wash once with saturated sodium chloride solution (3L).The organic phase was concentrated under reduced pressure to remove the solvent, added 2L n-heptane slurry, washed for 2h, filtered,After drying, 196 g of the compound represented by formula IV was obtained, the yield of the two-step reaction was 99.0%, and the purity: 97.3%.
References:
Jilin Huisheng Bio-pharmaceutical Co., Ltd.;Beijing Huizhiheng Biological Technology Co., Ltd.;Gao Binglei CN112047915, 2020, A Location in patent:Paragraph 0104-0106
1204220-63-2
7 suppliers
inquiry

864070-37-1
95 suppliers
inquiry
912917-85-2
21 suppliers
inquiry

864070-37-1
95 suppliers
inquiry
![D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]-](/CAS/20210111/GIF/912917-83-0.gif)
912917-83-0
2 suppliers
inquiry

864070-37-1
95 suppliers
inquiry
461432-26-8
715 suppliers
$5.00/5mg

864070-37-1
95 suppliers
inquiry