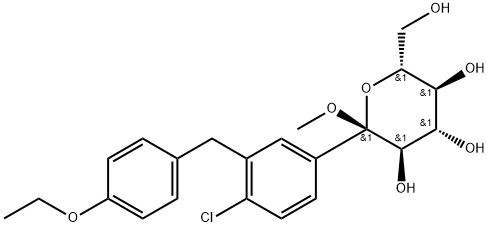
(2S,3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxyMethyl)-2-Methoxytetrahydro-2H-pyran-3,4,5-triol synthesis
- Product Name:(2S,3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxyMethyl)-2-Methoxytetrahydro-2H-pyran-3,4,5-triol
- CAS Number:714269-57-5
- Molecular formula:C22H27ClO7
- Molecular Weight:438.9

67-56-1
790 suppliers
$9.00/25ml

714269-57-5
194 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-bromo-1-chloro-2-[(4-ethoxyphenyl)methyl]benzenewith n-butyllithium in tetrahydrofuran;hexane;toluene at -30;
Stage #2: (3R,4S,5R,6R)-3,4,5-tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one in tetrahydrofuran;hexane;toluene at -30 - -24;
Stage #3: methanol in tetrahydrofuran;hexane;toluene at -10 - 35; for 23 h;Acidic conditions;
Steps:
16
Example 16; A reaction scheme similar to that shown in Scheme IVA and FIG. 22 was employed. A -30° C. chiller for the lithiation reactor 5 jacketed static mixer 5) was set up. A -30° C. chiller for the coupling reactor 22 jacketed static mixer 22) and a pre-cooling heat exchanger (not shown in FIG. 22) for the compound D/toluene feed was set up.; Continuous Lithiation; The two feeds of E/THF/toluene (2.74 ml/min) and Q, namely, n-BuLi in hexane (0.41 ml/min), were mixed and combined through jacketed static mixer 5 (-30° C.). Before pumping the D/toluene feed, toluene (2.96 ml/min) was sent into the system as a make-up flow to maintain the overall flow constant at 6.1 ml/min. Samples at the outlet of the lithiation static mixer 5 for HPLC analysis were collected. Samples were taken before (a) the onset of the coupling reaction, and (b) after the collection of the reaction mixture into the MSA-MeOH reactor.; Continuous Coupling Reaction; The D/toluene feed (2.96 ml/min) was pre-cooled via a heat exchanger before mixing with the lithiation stream. The two streams namely G and D were mixed and combined through a jacketed static mixer 22 (between -24° C. and -30° C.). The reaction stream appeared yellowish in color. Samples were collected at the outlet of the mixer 22 for HPLC analysis. Samples were taken before and after the collection into the MSA-MeOH reactor 25.; Methyl Glycosidation; The coupling reaction stream 24 was fed to a 500-ml reactor 25 containing MSA and methanol or HCl/MeOH at <-10° C. with stirring. After the collection were finished, the reaction mixture was kept at <-10° C. with stirring for another hour. The reaction mixture was heated up to 35° C. The reaction was deemed complete (about 6 hrs) until HPLC analysis indicated that desilylated hemiketal H' RAP<0.3%. The reaction was cooled to room temperature (20° C.) and the reaction mixture was held for 16 hrs to form compound B.; Formation of Crystals of If; B was crystallized with 2-butyne-1,4-diol (J) in toluene/EtOAc to yield crystals of If.
References:
US2008/4336,2008,A1 Location in patent:Page/Page column 25-26
90-80-2
581 suppliers
$5.00/25g

714269-57-5
194 suppliers
inquiry