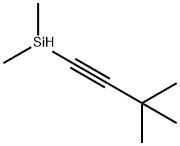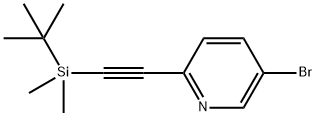
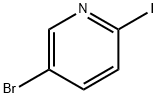
5-bromo-2-[(tert-butyl-dimethyl-silanyl)-ethynyl]-pyridine synthesis
- Product Name:5-bromo-2-[(tert-butyl-dimethyl-silanyl)-ethynyl]-pyridine
- CAS Number:866929-82-0
- Molecular formula:C13H18BrNSi
- Molecular Weight:296.28
624-28-2
726 suppliers
$5.00/5g
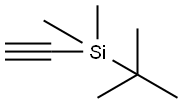
86318-61-8
119 suppliers
$22.39/250MG
![5-bromo-2-[(tert-butyl-dimethyl-silanyl)-ethynyl]-pyridine](/CAS/GIF/866929-82-0.gif)
866929-82-0
0 suppliers
inquiry
Yield:866929-82-0 100%
Reaction Conditions:
with triethylamine;bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide;N,N`-dimethylethylenediamine in tetrahydrofuran at -7 - 20; for 4 h;
Steps:
1.d 1d.
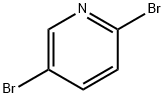
1d. 5-bromo-2-[(tert-butyldimethylsilanyl)ethynyl]pyridine Under an argon atmosphere, 0.80 g (4.20 mmol) of CuI and 2.90 g (4.13 mmol) of bis(triphenylphosphane)palladium (II) chloride are added to a solution of 49.90 g (201.0 mmol) of 2,5-dibromopyridine and 43.0 mL (225.6 mmol) of tert-butylethynyldimethylsilane in 500 mL of dry THF and 120 mL of triethylamine at -7° C. and the mixture is stirred for 30 minutes at 0° C. The reaction mixture is stirred for a further 3.5 hours at RT, then filtered, and the filtrate is evaporated down in vacuo. The residue is dissolved in 1 L of EtOAc, and the organic phase is washed with water and saturated NaCl solution, dried over sodium sulfate (Na2SO4), and evaporated down in vacuo. The crude product is further reacted without purification. Yield: 59.5 g (quant. yield); C13H18BrNSi (M=296.278); calc.: molpeak (M+H)+: 296/298 (Br); found: molpeak (M+H)+: 296/298 (Br); Rf value: 0.75 (silica gel, Cyc/EtOAc 8:1).
References:
US2005/267115,2005,A1 Location in patent:Page/Page column 22
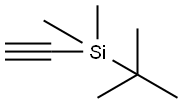
86318-61-8
119 suppliers
$22.39/250MG
223463-13-6
387 suppliers
$5.00/1g
![5-bromo-2-[(tert-butyl-dimethyl-silanyl)-ethynyl]-pyridine](/CAS/GIF/866929-82-0.gif)
866929-82-0
0 suppliers
inquiry