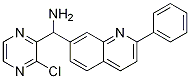
(3-chloropyrazin-2-yl)(2-phenylquinolin-7-yl)MethanaMine synthesis
- Product Name:(3-chloropyrazin-2-yl)(2-phenylquinolin-7-yl)MethanaMine
- CAS Number:867162-37-6
- Molecular formula:C20H15ClN4
- Molecular Weight:346.81
Yield:867162-37-6 83%
Reaction Conditions:
Stage #1: N-[bis(4-methoxyphenyl)methylidene]-1-(2-phenylquinolin-7-yl)methanaminewith sodium hexamethyldisilazane in tetrahydrofuran at -5; for 0.333333 h;Inert atmosphere;
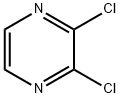
Stage #2: 2,3-dichloropyrazine in tetrahydrofuran; for 0.333333 h;Product distribution / selectivity;
Steps:
2
Example 2: C-(3-chloropyrazin-2-yl)-C-(2-phenylquinolin-7-yl)methylamine; N- [bis(4-methoxyphenyl)methylidene] - 1 -(2-phenylquinolin-7-yl)methanamine (100 mg, 0.22 mmol) was added to a flask and protected by nitrogen. THF (2 rnL) was added and a clear solution was obtained. The solution was cooled to -5 0C and then 1.0 M 1,1,1,3,3,3- hexamethyldisilazane, sodium salt in THF (0.26 mL, 0.26 mol) was added. After 20 min, 2,3- dichloropyrazine (36 mg, 0.24 mmol) in THF (1.0 mL) was added. After a further 20 min, 2M HCl (2 mL) was added and the mixture was stirred at room temperature for 10 min. The aqueous mixture was washed with DCM (3x) and then basifed to pH 10 with solid potassium carbonate. A white solid precipitated from the aqueous solution and the resulting suspension was extracted with DCM. The organic solution was washed with water, dried over sodium sulfate, filtered, and concentrated in vacuo to give a light yellow oil (67 mg). The yellow oil was further purified by silica gel chromatography (eluted with ethyl acetate/ methanol/ triethylamine, 10:0.5:1) to yield a colorless oil (63 mg, 83% yield).
References:
WO2010/123792,2010,A1 Location in patent:Page/Page column 9

867162-39-8
4 suppliers
inquiry

867162-37-6
5 suppliers
inquiry
![7-Quinolinemethanamine, N-[bis(4-methoxyphenyl)methylene]-2-phenyl-](/CAS/20210305/GIF/1253106-90-9.gif)