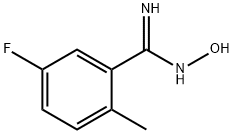
Benzenecarboximidamide, 5-fluoro-N-hydroxy-2-methyl- synthesis
- Product Name:Benzenecarboximidamide, 5-fluoro-N-hydroxy-2-methyl-
- CAS Number:872362-79-3
- Molecular formula:C8H9FN2O
- Molecular Weight:168.17
77532-79-7
190 suppliers
$10.00/1g

872362-79-3
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydroxylamine in ethanol at 90; for 12 h;
Steps:
Intermediate 1 (0461) 5-Fluoro-N-hydroxy-2-methylbenzenecarboximidamide
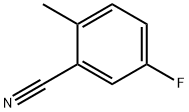
Intermediate 1 (0461) 5-Fluoro-N-hydroxy-2-methylbenzenecarboximidamide (0462) 5-Fluoro-2-methylbenzonitrile (1.01 g, 7.47 mmol) was dissolved in ethanol (15 mL) at ambient temperature. Hydroxylamine (0.550 mL, 8.97 mmol) was added and the reaction mixture warmed to 90° C. The reaction mixture was allowed to stir for 12 h at 90° C. The reaction mixture was allowed to cool and was concentrated under reduced pressure. The residue was purified by silica gel chromatography, eluting with a gradient of ethyl acetate:hexanes-0:100 to 35:65, to afford the title compound. MS: m/z=169.1 [M+H].
References:
US2017/275260,2017,A1 Location in patent:Paragraph 0461; 0462