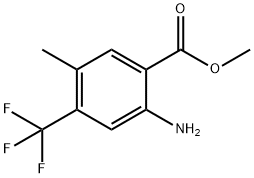
methyl 2-amino-5-methyl-4-(trifluoromethyl)benzoate synthesis
- Product Name:methyl 2-amino-5-methyl-4-(trifluoromethyl)benzoate
- CAS Number:872624-53-8
- Molecular formula:C10H10F3NO2
- Molecular Weight:233.19
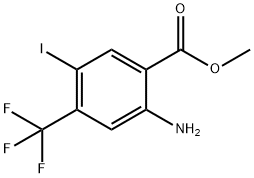
872624-52-7
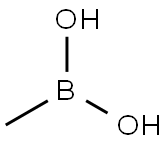
13061-96-6

872624-53-8
Step 4: Preparation of methyl 2-amino-5-methyl-4-(trifluoromethyl)benzoate To a solution of methyl 2-amino-5-iodo-4-(trifluoromethyl)benzoate (121 g, 351 mmol) in anhydrous 1,4-dioxane (2.5 L) under nitrogen protection was added sequentially cesium fluoride (184.3 g, 1.21 mol), methylboronic acid (63.7 g, 1.05 mol, 3 molar equivalents), and bis(diphenylphosphinoferrocene)palladium chloride (II ) (27.83 g, 35.1 mmol). The reaction mixture was heated and stirred at 80 °C for 3 hours. Upon completion of the reaction, which was cooled to room temperature, the reaction solution was partitioned between ethyl acetate (2.5 L) and water (2.5 L) and filtered through a Celite pad to remove the fine black suspension. The organic phase was separated and the aqueous phase was extracted with ethyl acetate (2 x 100 mL). The organic phases were combined, washed with brine (1 L) and dried over anhydrous magnesium sulfate. After filtration, it was concentrated under reduced pressure (45°C) to obtain a red oil. Purification by silica gel column chromatography with isohexane/ethyl acetate (9:1) as eluent gave methyl 2-amino-5-methyl-4-(trifluoromethyl)benzoate (75.25 g, 92% yield) as a light yellow crystalline solid.

872624-52-7
54 suppliers
$15.00/100mg

13061-96-6
304 suppliers
$6.00/1g

872624-53-8
44 suppliers
$36.00/100mg
Yield: 92%
Reaction Conditions:
with cesium fluoride;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 20 - 80; for 3 h;
Steps:
3.4
Step 4; Preparation of Methyl 2-amino-5-methyl-4-trifluoromethylbenzoate; Add cesium fluoride (184.3 g, 1.21 mol), methyl boronic acid (63.7 g, 1.05 mol, 3 molequiv.) andbis(diphenylphosphinoferrocene)palladium(II) chloride (27.83 g, 35.1 mmol)to a solution of methyl 2-amino-5-iodo-4-trifluoromethylbenzoate (121 g, 351 mmol) inanhydrous 1,4-dioxane (2.5 L) at room temperature under an atmosphere of nitrogen andheat the mixture at 80 °C for 3 h. Allow the mixture to cool to room temperature thenpartition between ethyl acetate (2.5 L) and water (2.5 L) and filter through a pad ofCelite to remove the fine black suspension. Extract the aqueous phase with ethylacetate (2 x 100 mL) and wash the combined organic extracts with brine (1 L). Dry overanhydrous magnesium sulfate, filter and remove the solvent under reduced pressure at 45°C to give a red oil. Purify the residue by column chromatography on silica gel, elutingwith isohexane/ethyl acetate (9:1), to give the title compound as a pale yellow crystallinesolid (75.25 g, 92%).
References:
ELI LILLY AND COMPANY WO2006/2342, 2006, A1 Location in patent:Page/Page column 56-57