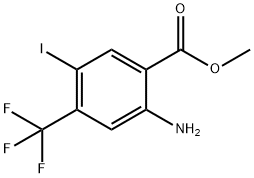
methyl 2-amino-5-iodo-4-(trifluoromethyl)benzoate synthesis
- Product Name:methyl 2-amino-5-iodo-4-(trifluoromethyl)benzoate
- CAS Number:872624-52-7
- Molecular formula:C9H7F3INO2
- Molecular Weight:345.06
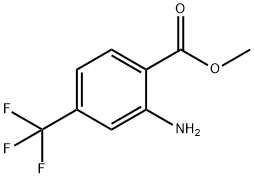
61500-87-6

872624-52-7
Step 3: Preparation of methyl 2-amino-5-iodo-4-(trifluoromethyl)benzoate; A solution of methyl 2-amino-4-(trifluoromethyl)benzoate (178 g, 812 mmol) in ethanol (3.3 L) was slowly added to a suspension in ethanol (5 L) containing iodine (206.1 g, 812 mmol) and silver(II) sulfate (253 g, 812 mmol). . The reaction was stirred at room temperature and under nitrogen protection for 2 hours. After completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad and the pad was washed with ethanol (2 L). The filtrate and washings were combined and concentrated under reduced pressure at 40°C to remove the solvent. The resulting residue was dissolved in ethyl acetate (7.5 L) and washed sequentially with saturated sodium bicarbonate solution (3 x 1.5 L), water (3 x 1.5 L) and brine (2 L). The organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to afford methyl 2-amino-5-iodo-4-(trifluoromethyl)benzoate as a light brown crystalline solid (276.0 g, 99% yield).

61500-87-6
119 suppliers
$19.00/1g

872624-52-7
54 suppliers
$15.00/100mg
Yield:872624-52-7 99%
Reaction Conditions:
with iodine;silver sulfate in ethanol at 20; for 2 h;Product distribution / selectivity;
Steps:
3.3
Step 3; Preparation of methyl 2-amino-5-iodo-4-trifluoromethylbenzoate; Add a solution of methyl 2-amino-4-trifluoromethylbenzoate (178 g, 812 mmol) inethanol (3.3 L) to a suspension of iodine (206.1 g, 812 mmol) and silver(II) sulfate (253g, 812 mmol) in ethanol (5 L) at room temperature under an atmosphere of nitrogen andstir for 2 h. Filter the suspension through a pad of a Celite, wash the pad withadditional ethanol (2 L) and remove the solvents from the combined filtrates underreduced pressure at 40 °C. Dissolve the residue in ethyl acetate (7.5 L) and wash withsaturated sodium bicarbonate solution (3 x 1.5 L), water (3 x 1.5 L) and brine (2 L). Drythe organic phase over anhydrous magnesium sulfate, filter and remove the solvent underreduced pressure to give the title compound as a pale brown crystalline solid (276.0 g,99%).
References:
WO2006/2342,2006,A1 Location in patent:Page/Page column 56
402-13-1
171 suppliers
$18.00/5g

872624-52-7
54 suppliers
$15.00/100mg
320-94-5
233 suppliers
$9.00/1g

872624-52-7
54 suppliers
$15.00/100mg