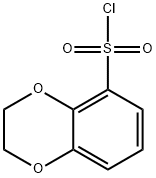
2,3-DIHYDRO-1,4-BENZODIOXINE-5-SULFONYL CHLORIDE,97% synthesis
- Product Name:2,3-DIHYDRO-1,4-BENZODIOXINE-5-SULFONYL CHLORIDE,97%
- CAS Number:87474-15-5
- Molecular formula:C8H7ClO4S
- Molecular Weight:234.66
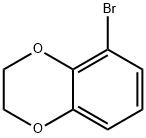
58328-39-5
78 suppliers
$36.00/100mg

87474-15-5
21 suppliers
$121.00/250mg
Yield:87474-15-5 69%
Reaction Conditions:
Stage #1: 5-bromo-2,3-dihydrobenzo[1,4]dioxinwith n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2: with sulfur dioxide in tetrahydrofuran;hexane; for 0.166667 h;
Stage #3: with N-chloro-succinimide in acetic acid at -78 - 20; for 1.25 h;
Steps:
158 Example 158
Preparation of 2,3-Methylenedioxy-benzene sulphonyl chloride
To 2,3-Methylenedioxy-bromobenzene (2.43 g, 0.012 mole) in 20 mL of THF at -78° C., was added slowly n-BuLi (4.84 mL, 2.5 M in hexane) was slowly added. The reaction was stirred at for 1 hour and then SO2 (g) was bubbled through the solution for 10 minutes. The reaction was warmed to room temperature, poured into 200 mL of ether, filtered, and dried under vacuum, to afford the lithio salt (2.1 g, 91 % yield). The lithio salt (2.1 g) was dissolved in 20 mL of glacial acetic acid at 15° C. NCS (1.61 g, 0.013 moles) was added over 15 minutes. The mixture was warmed to room temperature and stirred for 1 hour. The solvent was removed under vacuum and the residue was dissolved in 100 mL of methylene chloride, washed with 50 mL of sat. sodium bicarbonate, dried over magnesium sulfate and the solvent was removed under vacuum. The residue was chromatographed on 100 g of silica gel, eluted with methylene chloride and the fractions were combined and evaporated under reduced pressure to yield 2,3-methylenedioxy-benzene sulphonyl chloride (1.7 g, 69 % yield).
References:
US6342504,2002,B1 Location in patent:Page column 123-124
14381-51-2
161 suppliers
$11.00/1g

87474-15-5
21 suppliers
$121.00/250mg