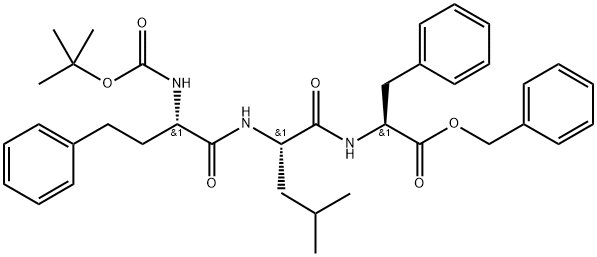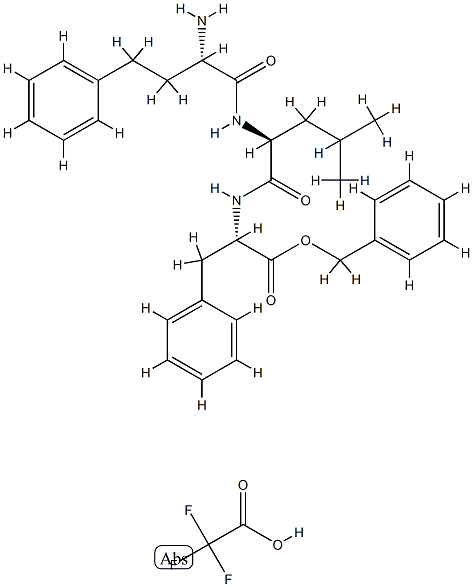
L-Phenylalanine, (αS)-α-aminobenzenebutanoyl-L-leucyl-, phenylmethyl ester (monotrifluoroacetate) synthesis
- Product Name:L-Phenylalanine, (αS)-α-aminobenzenebutanoyl-L-leucyl-, phenylmethyl ester (monotrifluoroacetate)
- CAS Number:875309-83-4
- Molecular formula:C34H40F3N3O6
- Molecular Weight:643.6931
Yield:-
Reaction Conditions:
in dichloromethane at 20; for 1 h;
Steps:
W
Compound (V) (0.25 g, 0.39 mmol) was mixed with 12 mL of TFA/DCM (80%) and was stirred at room temperature for 1 hr at which time the mixture was concentrated and placed under high vacuum for 2 hr giving the TFA salt of the tri-peptide amine. The crude amine salt was dissolved in 6 mL DMF and 2-morpholino acetic acid (0.074 g, 0.507 mmol) was added followed by DIEA (0.504 g, 0.68 mL, 3.90 mmol). The mixture was cooled to 0° C. in an ice bath and PyBOP (0.32 g, 0.62 mmol) was added and stirred under an atmosphere of argon while warming to room temperature overnight. The mixture was diluted with brine (50 mL) and extracted with EtOAc (5×20 mL). The organic layers were combined, washed with sat. NaHCO3 (5×15 mL) and brine (1×25 mL) and dried over MgSO4. The MgSO4 was removed by filtration and the volatiles removed under reduced pressure to give the intermediate ester (0.195 g). To (0.150g, 0.23 mmol) of the intermediate ester was added 10% Pd/C (0.05g) followed by 5 mL of 1:1 mixture of MeOH and EtOAc and the mixture was placed under an atmosphere of hydrogen. After 2 hr, the contents were filtered through a plug of Celite and concentrated under vacuum to give (W) (0.12 g).
References:
US2005/245435,2005,A1 Location in patent:Page/Page column 31-32

70637-28-4
12 suppliers
inquiry

875309-83-4
19 suppliers
inquiry
13139-15-6
446 suppliers
$7.00/5g

875309-83-4
19 suppliers
inquiry
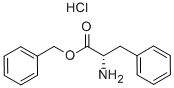
2462-32-0
237 suppliers
$10.00/1g

875309-83-4
19 suppliers
inquiry