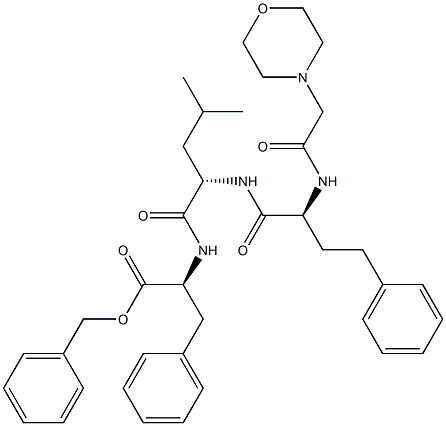
(S)-benzyl 2-((S)-4-methyl-2-((S)-2-(2-morpho lino acetamido)-4-phenylbutanamido)pentanamido)-3-phenylpropanoate synthesis
- Product Name:(S)-benzyl 2-((S)-4-methyl-2-((S)-2-(2-morpho lino acetamido)-4-phenylbutanamido)pentanamido)-3-phenylpropanoate
- CAS Number:875309-92-5
- Molecular formula:C38H48N4O6
- Molecular Weight:656.81
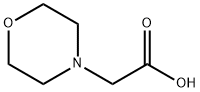
3235-69-6
218 suppliers
$6.00/250mg
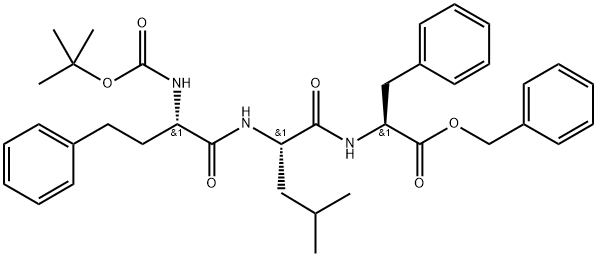
868540-15-2
84 suppliers
inquiry

875309-92-5
22 suppliers
inquiry
Yield:875309-92-5 94 g
Reaction Conditions:
Stage #1: (S)-benzyl 2-((S)-2-((S)-2-(tert-butoxycarbonylamino)-4-phenylbutanamido)-4-methylpentanamido)-3-phenylpropanoatewith trifluoroacetic acid in dichloromethane at 2 - 30; for 2 h;
Stage #2: morpholin-4-ylacetic acidwith benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate;benzotriazol-1-ol;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 2 - 30;Temperature;Reagent/catalyst;
Steps:
6 EXAMPLE 6: Preparation of compound of Formula XIV
EXAMPLE 6: Preparation of compound of Formula XIV A mixture of methylene chloride (60 ml) and trifluoro acetic acid (240 ml) was allowed to cool to 2-6°C. To the reaction mass compound of Formula VII (lOOg, leq) was added at same temperature. Then the reaction mass was heated to 25-30°C and stirred for 2 hr at same temperature. To the reaction mass methylene chloride (1.51it) was added and pH adjusted to 7-8 with 20% sodium carbonate at 25-30°C and stirred for 20 min at same temperature. Separated the organic layer and washed with water (300 ml) and 10% sodium chloride (300 ml) sequentially. Organic layer was separated and concentrated under vacuum at 25-35°C to obtain residue. The obtained residue was dissolved in dimethyl formamide (500 ml) and allowed to cool to 2-6°C. To the reaction mass HOBt (2.2g, O. leq), PyBOP (99.2g, 1.2eq), morpholine acetic acid (29.5g, 1.2eq) and diisopropyl ethyl amine (82. lg, 4eq) were slowly added sequentially at 2-6°C. Then the reaction mass was heated to 25-30°C and stirred for 2-3 hr at same temperature. After completion of the reaction, reaction mass was quenched in to water (51it) at 25-30°C and stirred for 2 hr at same temperature and the solid precipitated was filtered, washed with water (500 ml) and dried to get the title compound. Yield: 94 g; Chemical purity by HPLC: 97.6%; HOBt: 0.35%, PyBOP: 0.01% and tris(pyrrolidino phosphine) oxide: 0.04% by HPLC.
References:
WO2016/185450,2016,A1 Location in patent:Page/Page column 64

3235-69-6
218 suppliers
$6.00/250mg
875309-83-4
19 suppliers
inquiry

875309-92-5
22 suppliers
inquiry
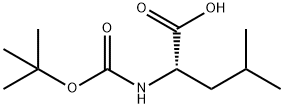
13139-15-6
447 suppliers
$7.00/5g

875309-92-5
22 suppliers
inquiry
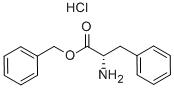
2462-32-0
237 suppliers
$10.00/1g

875309-92-5
22 suppliers
inquiry

70637-28-4
12 suppliers
inquiry

875309-92-5
22 suppliers
inquiry