
Ethyl 2-(4-(hydroxymethyl)piperidin-1-yl)pyrimidine-5-carboxylate synthesis
- Product Name:Ethyl 2-(4-(hydroxymethyl)piperidin-1-yl)pyrimidine-5-carboxylate
- CAS Number:875318-46-0
- Molecular formula:C13H19N3O3
- Molecular Weight:265.31
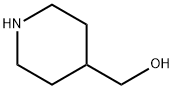
6457-49-4
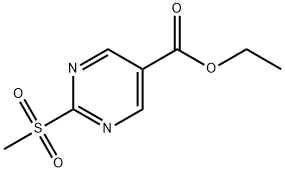
148550-51-0

875318-46-0
Stage 1 - Coupling Reaction: Under nitrogen protection, piperidine-4-methanol (2.48 g, 21.55 mmol) was stirred with K2CO3 (8.9 g, 64.65 mmol) in a 1:1 solvent mixture of DMF/MeCN (20 mL) for 10 min at room temperature. Subsequently, ethyl 2-methylsulfonyl-5-pyrimidinecarboxylate (5 g, 21.55 mmol) was added and the reaction continued to be stirred for 20 min. After completion of the reaction, the reaction mixture was diluted with deionized water (100 mL) and extracted with ethyl acetate (2 x 100 mL). The organic layers were combined, dried with anhydrous MgSO4 and concentrated under reduced pressure to remove the solvent to afford the orange solid product ethyl 2-(4-hydroxymethylpiperidin-1-yl)pyrimidine-5-carboxylate (5.70 g, 99% yield). Mass spectrometry analysis showed m/z = 266 [M + H]+. The product was used directly in subsequent steps without further purification.

6457-49-4
355 suppliers
$18.00/25g

148550-51-0
60 suppliers
$193.59/250mg

875318-46-0
30 suppliers
$16.00/100mg
Yield:875318-46-0 99%
Reaction Conditions:
Stage #1: 4-piperidinemethanolwith potassium carbonate in N,N-dimethyl-formamide;acetonitrile at 20; for 0.166667 h;
Stage #2: ethyl 2-(methylsulfonyl)pyrimidine-5-carboxylate in N,N-dimethyl-formamide;acetonitrile; for 0.333333 h;
Steps:
3.1
Stage 1 - Coupling; Piperidin-4yl-methanol (2.48g, 21.55mmol) was stirred in 1 :1 DMF/MeCN (2OmL) with K2CO3 (8.9g, 64.65mmol) for 10 minutes at RT under a nitrogen atmosphere. Intermediate F (5g, 21.55mmol) was then added and the reaction allowed to stir for 20 minutes. It was then diluted with H2O (10OmL) and extracted with EtOAc (2 x 10OmL). The combined organic layers were dried (MgSO4) and the solvent removed in vacuo to give the product as an orange solid which was used in the next step without further purification (5.7Og, 99%). m/z = 266 [M+H]+.
References:
WO2008/53131,2008,A1 Location in patent:Page/Page column 51; 53

6457-49-4
355 suppliers
$18.00/25g
89793-12-4
234 suppliers
$6.00/100mg

875318-46-0
30 suppliers
$16.00/100mg
5909-24-0
418 suppliers
$6.00/5g

875318-46-0
30 suppliers
$16.00/100mg
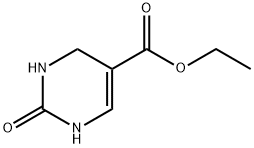
33458-27-4
28 suppliers
$152.00/500mg

875318-46-0
30 suppliers
$16.00/100mg
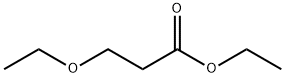
763-69-9
352 suppliers
$14.00/25mL

875318-46-0
30 suppliers
$16.00/100mg