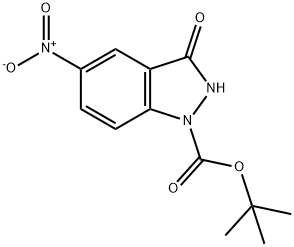
1H-Indazole-1-carboxylic acid, 2,3-dihydro-5-nitro-3-oxo-, 1,1-diMethylethyl ester synthesis
- Product Name:1H-Indazole-1-carboxylic acid, 2,3-dihydro-5-nitro-3-oxo-, 1,1-diMethylethyl ester
- CAS Number:876343-67-8
- Molecular formula:C12H13N3O5
- Molecular Weight:279.25
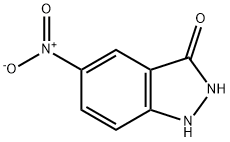
61346-19-8
58 suppliers
inquiry

24424-99-5
815 suppliers
$13.50/25G

876343-67-8
10 suppliers
inquiry
Yield:876343-67-8 61%
Reaction Conditions:
Stage #1: 5-nitroindazolin-3-onewith sodium hydrogencarbonate in water; for 0.5 h;
Stage #2: di-tert-butyl dicarbonate in water at 45;
Steps:
19.C
19B (0.500 g, 2.319 mmol) was mixed with water (10 mL). To this solution/suspension sodium bicarbonate (0.214 g, 2.55 mmol) was added portionwise. The mixture was stirred for 30 min. BOC-Anhydride (0.700 mL, 3.01 mmol) was added, the mixture was heated to 45 0C and stirred at this temperature for 30 min. Additional portions of sodium bicarbonate (totaling 1.5 g) and BoC2O (totaling 1 g) were added and the mixture was stirred overnight at 45 0C. The reaction mixture was diluted with water (100 mL), and extracted with EtOAc (3 x 25 mL). The combined organic phase was washed with water (2 x 50 mL), brine (1 x 50 mL) and dried (Na2SO4). After filtration, the solvent removed under reduced pressure and the resulting solid was washed with ether/hexanes (3x) and dried to give 19C (0.395 g, 1.415 mmol, 61.0 % yield) as a yellowish solid. LC-MS: (Phenom. Luna C18 30x4.6mm 5u; sol. A 10% MeCN - 90% H2O - 0.1% TFA; sol. B 90% MeCN - 10% H2O - 0.1% TFA; wavelength 220 nm; flow rate 5 mL/min; gradient time 2 min; start % B = 0%, final % B = 100%) 1.217 min, [M+lf = 280.02. Purity >95%. 1H-NMR: (400 MHz, DMSO-d6) δ ppm 1.61 (s, 9 H) 8.13 (d, J=9.34 Hz, 1 H) 8.40 (dd, J=9.34, 2.20 Hz, 1 H) 8.57 (d, J=2.20 Hz, 1 H) 12.60 (s, 1 H). 13C-NMR: (101 MHz, DMSO- d6) δ ppm 27.69, 84.56, 114.97, 116.99, 117.24, 124.60, 143.02, 148.21, 158.48.
References:
WO2008/79759,2008,A1 Location in patent:Page/Page column 130