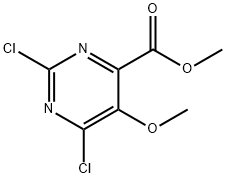
Methyl 2,6-dichloro-5-methoxypyrimidine-4-carboxylate synthesis
- Product Name:Methyl 2,6-dichloro-5-methoxypyrimidine-4-carboxylate
- CAS Number:878650-31-8
- Molecular formula:C7H6Cl2N2O3
- Molecular Weight:237.04

67-56-1
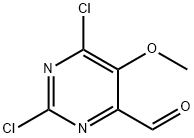
1446253-05-9

878650-31-8
Example 5 Preparation of methyl 2,6-dichloro-5-methoxypyrimidine-4-carboxylate: 2,6-dichloro-5-methoxypyrimidine-4-carboxaldehyde (50 g, 0.24 mol) was dissolved in a solvent mixture of methanol (1 L) and water (60 mL). Sodium bicarbonate (400 g) was added to this solution. A methanol/water (600 mL, v/v=9:1) solution of 2M bromine (192 g, 1.2 mol) was slowly added dropwise to the pyrimidine solution over a period of 45 min at 0°C while maintaining stirring. After the dropwise addition was completed, stirring was continued at the same temperature for 1 hour. Subsequently, the reaction mixture was warmed to room temperature and stirred for 4 hours. Upon completion of the reaction, the mixture was slowly poured into a mixture containing crushed ice (2 L), sodium bisulfite (50 g) and sodium chloride (200 g). The product was extracted with ethyl acetate (1L x 2), the organic layers were combined and dried over anhydrous sodium sulfate and filtered. The solvent was removed by concentration under reduced pressure to give a viscous mass, which was left to cure to give methyl 2,6-dichloro-5-methoxypyrimidine-4-carboxylate (50.8 g, 87% yield): ESIMS m/z 238 ([M+H]+).

67-56-1
790 suppliers
$9.00/25ml

1446253-05-9
5 suppliers
inquiry

878650-31-8
44 suppliers
$15.00/100mg
Yield:878650-31-8 87%
Reaction Conditions:
with bromine;sodium hydrogencarbonate in water at 0 - 20; for 5.75 h;
Steps:
5 Preparation of methyl 2,6-dichloro-5-methoxy-pyrimidine-4-carboxylate
Example 5
Preparation of methyl 2,6-dichloro-5-methoxy-pyrimidine-4-carboxylate
A solution of 2,6-dichloro-5-methoxy-pyrimidine-4-carbaldehyde (50 g, 0.24 mol) in methanol (1 L) and water (60 mL) was prepared.
To the solution, sodium bicarbonate (400 g) was added.
A 2 M solution of bromine (192 g, 1.2 mol) in methanol/water (600 mL, 9:1) was added dropwise to the pyrimidine solution over 45 minutes (min) at 0° C. while stirring the mixture.
The stirring was continued at the same temperature for 1 h.
Later, the mixture was stirred at room temperature for 4 h.
While stirring, the reaction mixture was thereafter poured onto a mixture of crushed ice (2 L), sodium bisulfite (50 g), and sodium chloride (200 g).
The product was extracted with ethyl acetate (1 L*2), and the combined organic layer was dried over sodium sulfate and filtered.
Evaporation of the solvent under reduced pressure produced a thick material, which solidified on long standing to afford the title compound (50.8 g, 87%): ESIMS m/z 238 ([M+H]+).
References:
US2014/274703,2014,A1 Location in patent:Paragraph 0197; 0198

1446253-05-9
5 suppliers
inquiry

878650-31-8
44 suppliers
$15.00/100mg
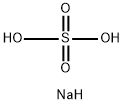
7681-38-1
266 suppliers
$18.00/25g

1446253-05-9
5 suppliers
inquiry

878650-31-8
44 suppliers
$15.00/100mg
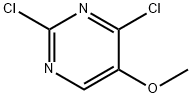
19646-07-2
334 suppliers
$15.00/1g

878650-31-8
44 suppliers
$15.00/100mg
1446253-03-7
7 suppliers
inquiry

878650-31-8
44 suppliers
$15.00/100mg