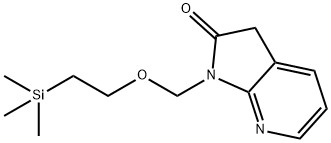
1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one synthesis
- Product Name:1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one
- CAS Number:879132-48-6
- Molecular formula:C13H20N2O2Si
- Molecular Weight:264.4
![1,3-DIHYDRO-2H-PYRROLO[2,3-B]PYRIDINE-2-ONE](/CAS/GIF/5654-97-7.gif)
5654-97-7
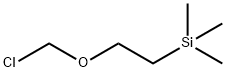
76513-69-4
![1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](/CAS/GIF/879132-48-6.gif)
879132-48-6
Under nitrogen protection, 7-aza-indol-2-one (2.00 g, 14.9 mmol) was dissolved in a solvent mixture of DMF (30 mL) and THF (30 mL) and cooled to 0 °C. Sodium hydride (0.600 g, 15.0 mmol) dispersed in 60% mineral oil was slowly added and kept stirring at 0 °C for 1 hour. Subsequently, 2-(trimethylsilyl)ethoxymethyl chloride (3.17 mL, 17.9 mmol) was added dropwise over 3 minutes, the ice bath was withdrawn, and the reaction mixture was allowed to warm up naturally to room temperature and stirring was continued for 21 hours. Upon completion of the reaction, the mixture was poured into water (50 mL) and ethyl acetate (50 mL) for partitioning. The aqueous phase was extracted with ethyl acetate (2 x 50 mL) and the organic phases were combined and washed sequentially with water (2 x 50 mL) and brine (50 mL). The organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated, and purified by Biotage Horizon fast column chromatography (eluent: 25% ethyl acetate/hexane) to afford 1-[[2-(trimethylsilyl)ethoxy]methyl]-1H-pyrrolo[2,3-b]pyridin-2(3H)-one (2.26 g) as a viscous, orange-colored oil.LC-MS analysis Conditions: retention time = 3.49 min; m/z = 265.20 [M+H]+ (column: Phenomenex C18 50×2.0 mm 3 μm; mobile phase A: 90% water:10% methanol:0.1% trifluoroacetic acid; mobile phase B: 10% water:90% methanol:0.1% trifluoroacetic acid; flow rate = 0.8 mL/min; gradient program: (starting B-phase ratio = 0%, final B-phase ratio = 100%, gradient time = 4 min, then held at 100% B-phase for 1 min; column temperature = 40 °C; detection wavelength = 220 nm).1H NMR (400 MHz, DMSO-d6) δ 8.15 (d, J=5.0 Hz, 1H), 7.65 (d, J=7.3 Hz, 1H), 7.05 ( dd, J=7.2,5.4 Hz, 1H), 5.07 (s, 2H), 3.69 (s, 2H), 3.59 (t, J=8.0 Hz, 2H), 0.85 (t, J=8.0 Hz, 2H), -0.06 (s, 9H).
![1,3-DIHYDRO-2H-PYRROLO[2,3-B]PYRIDINE-2-ONE](/CAS/GIF/5654-97-7.gif)
5654-97-7
127 suppliers
$38.00/1g

76513-69-4
358 suppliers
$6.00/1g
![1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](/CAS/GIF/879132-48-6.gif)
879132-48-6
72 suppliers
$13.00/100mg
Yield: 2.26 g
Reaction Conditions:
Stage #1:7-azaoxindole with sodium hydride in tetrahydrofuran;N,N-dimethyl-formamide;mineral oil at 0; for 1 h;Inert atmosphere;
Stage #2:(2-trimethylethylsilylethoxy)methyl chloride in tetrahydrofuran;N,N-dimethyl-formamide;mineral oil at 20; for 21 h;Inert atmosphere;
Steps:
GW-23.1
To a solution of 1H-pyrrolo[2,3-b]pyridin-2(3H)-one (2.00 g, 14.9 mmol) in DMF (30 mL) and THF (30 mL) under nitrogen at 0 °C was added sodium hydride, 60% in mineral oil (0.600 g, 15.0 mmol). The reaction mixture was stirred at 0 °C for 1 h. Then (2- (chloromethoxy)ethyl)trimethylsilane (3.17 mL, 17.9 mmol) was added dropwise over 3 min and the reaction was allowed to warm up to rt without removing the cold bath over 21 h. The reaction mixture was partitioned between water (50 mL) and EtOAc (50 mL), the aqueous was extracted with EtOAc (2 x 50 mL), the combined organic component was washed with water (2 x 50 mL), brine (50 mL), dried over MgS04, filtered, concentrated and purified with a Biotage Horizon (25% EtOAc/hexanes) to afford the title compound (2.26 g ) as viscous orange oil. LC-MS retention time = 3.49 min; m/z = 265.20 [M+H]+. (Column: Phenomenex C18 50 x 2.0 mm 3 μιη. Solvent A = 90% Water : 10% MeOH : 0.1% TFA. Solvent B = 10% Water : 90% MeOH : 0.1% TFA. Flow Rate = 0.8 mL/min. Start % B = 0. Final % B = 100. Gradient Time = 4 minutes, then a l-minute hold at l00% B. Oven temperature = 40 °C. Wavelength = 220 nm). 1H NMR (400 MHZ, DMSO-d6) δ 8.15 (d, J=5.0 Hz, 1H), 7.65 (d, J=7.3 Hz, 1H), 7.05 (dd, J=7.2, 5.4 Hz, 1H), 5.07 (s, 2H), 3.69 (s, 2H), 3.59 (t, J=8.0 Hz, 2H), 0.85 (t, J=8.0 Hz, 2H), -0.06 (s, 9H).
References:
BRISTOL-MYERS SQUIBB COMPANY;BENDER, John A.;GENTLES, Robert G.;PENDRI, Annapurna;WANG, Alan Xiangdong;MEANWELL, Nicholas A.;BENO, Brett R.;FRIDELL, Robert A.;BELEMA, Makonen;NGUYEN, Van N.;YANG, Zhong;WANG, Gan;KUMARAVEL, Selvakumar;THANGATHIRUPATHY, Srinivasan;BORA, Rajesh Onkardas;HOLEHATTI, Shilpa Maheshwarappa;METTU, Mallikarjuna Rao;PANDA, Manoranjan WO2016/172424, 2016, A1 Location in patent:Page/Page column 90; 91
![3,3-dibroMo-1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](/CAS/GIF/879132-47-5.gif)
879132-47-5
18 suppliers
$557.00/1g
![1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](/CAS/GIF/879132-48-6.gif)
879132-48-6
72 suppliers
$13.00/100mg
![1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/879132-46-4.gif)
879132-46-4
34 suppliers
$55.00/1g
![1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](/CAS/GIF/879132-48-6.gif)
879132-48-6
72 suppliers
$13.00/100mg

76513-69-4
358 suppliers
$6.00/1g
![1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](/CAS/GIF/879132-48-6.gif)
879132-48-6
72 suppliers
$13.00/100mg
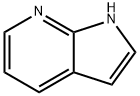
271-63-6
573 suppliers
$9.00/5g
![1-((2-(triMethylsilyl)ethoxy)Methyl)-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](/CAS/GIF/879132-48-6.gif)
879132-48-6
72 suppliers
$13.00/100mg