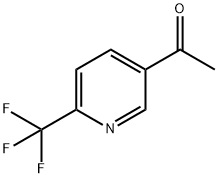
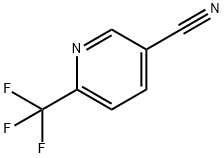
1-(6-(trifluoroMethyl)pyridin-3-yl)ethanone synthesis
- Product Name:1-(6-(trifluoroMethyl)pyridin-3-yl)ethanone
- CAS Number:358780-14-0
- Molecular formula:C8H6F3NO
- Molecular Weight:189.13
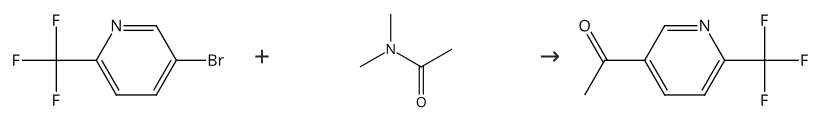
358780-13-9
97 suppliers
$45.00/100mg
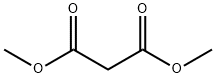
108-59-8
647 suppliers
$5.00/25g

358780-14-0
125 suppliers
$32.00/100mg
Yield:358780-14-0 79%
Reaction Conditions:
Stage #1:malonic acid dimethyl ester with triethylamine;magnesium chloride in toluene for 2.5 h;light cooling;
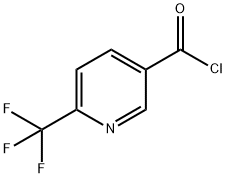
Stage #2:6-(trifluoromethyl)nicotinoyl chloride in toluene for 2 h;Cooling;
Stage #3: with hydrogenchloride;watermore than 3 stages;
Steps:
6.i
i) l-(6-Trifluoromethyl-pyridin-3-yl)-ethanone. Magnesium chloride 3.18 g (33.4 mmol) was suspended in 160 ml toluene. After the addition of triethylamine 16.63 ml (119.3 mmol) and malonic acid dimethyl ester 6.58 ml (57.3 mmol) the reaction mixture was stirred for 2.5 hours under light cooling. 6-Trifluoromethyl-nicotinoyl chloride was dissolved in 15 ml toluene and added dropwise under cooling to the reaction mixture. After stirring for 2 hours the mixture was acidified with cone, hydrochloric acid (15 ml). A white precipitate was formed which was dissolved by the addition of water. The water was separated and extracted one time with ethyl acetate and one time with dichloromethane. The combined organic layers were evaporated and the residue was dissolved in 52 ml dimethyl sulfoxide (DMSO) and 2 ml water. The obtained solution was stirred at155 0C for 2.5 hours. After cooling and the addition of 100 ml ice water it was stirred for 15 minutes. The product was isolated by suction and washed with ice water (3 x 15 ml). The solid was dried at 400C to give 7.11 g (79%) of the title compound as white solid. MS: 189.90 (ESI+)1H-NMR (400 MHz, [D6]DMSO): δ = 2.69 (s, 3H, CH3), 8.08 (d, IH, 5-H-pyridine), 8.56 (d, IH, 4-H-pyridine), 9.26 (s, IH, 2-H-pyridine)
References:
F. HOFFMANN-LA ROCHE AG WO2008/34579, 2008, A1 Location in patent:Page/Page column 24-25
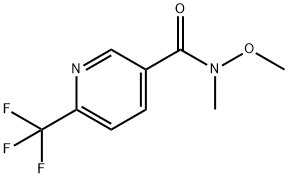
416852-53-4
10 suppliers
$450.00/1 g

75-16-1
279 suppliers
$12.00/10ml

358780-14-0
125 suppliers
$32.00/100mg
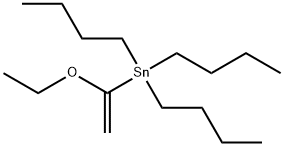
97674-02-7
232 suppliers
$15.00/1 g
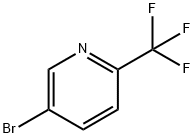
436799-32-5
328 suppliers
$5.00/250mg

358780-14-0
125 suppliers
$32.00/100mg
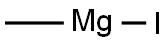
917-64-6
148 suppliers
$45.00/10ml

416852-53-4
10 suppliers
$450.00/1 g

358780-14-0
125 suppliers
$32.00/100mg

75-16-1
279 suppliers
$12.00/10ml
216431-85-5
208 suppliers
$8.00/1g

358780-14-0
125 suppliers
$32.00/100mg