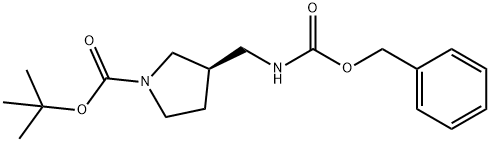
tert-butyl (3R)-3-({[(benzyloxy)carbonyl]amino}methyl)pyrrolidine-1-carboxylate synthesis
- Product Name:tert-butyl (3R)-3-({[(benzyloxy)carbonyl]amino}methyl)pyrrolidine-1-carboxylate
- CAS Number:879275-54-4
- Molecular formula:C18H26N2O4
- Molecular Weight:334.41
199174-29-3
152 suppliers
$38.00/100mg

501-53-1
409 suppliers
$10.00/1g
![tert-butyl (3R)-3-({[(benzyloxy)carbonyl]amino}methyl)pyrrolidine-1-carboxylate](/CAS/20180703/GIF/879275-54-4.gif)
879275-54-4
10 suppliers
inquiry
Yield:879275-54-4 77%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0; for 4 h;
Steps:
306
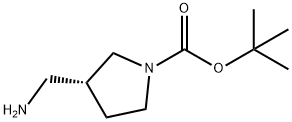
A solution of (R)-tert-butyl 3-(aminomethyl)pyrrolidine-l-carboxylate (1.0 g, 5.0mmol) and 50 mL THF was cooled in an ice bath. To this mixture was added benzylchloro'forma'te' (W?M?5S mmol)/lWlbwed by triethylamine (1.39 mL, 10 mmol). After removing the ice bath, the reaction was stirred for 4 h. The mixture was poured into ice water and extracted with EtOAc. The organic extracts were washed with water, dried over Na2SC>4, filtered, and concentrated. Purification via silica gel chromatography using EtOAc in hexanes (0^40%) gave benzyl ((i?)-l-(tert-butoxycarbonyl)pyrrolidin-3-yl)methylcarbamate (1.29 g, 77%) as a colorless oil. .H NMR (400 MHz, CDC13) 5 7.39-7.28 (m, 5H), 5.10 (s, 2H), 4.87 (s, 1H), 3.52-2.95 (m, 6H), 2.41-2.35 (m, 1H), 2.10-1.79 (m, 2H), 1.45 (s, 9H).
References:
WO2006/28904,2006,A1 Location in patent:Page/Page column 331-332