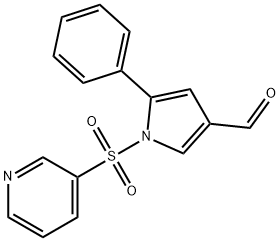
5-phenyl-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde synthesis
- Product Name:5-phenyl-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde
- CAS Number:881676-90-0
- Molecular formula:C16H12N2O3S
- Molecular Weight:312.34
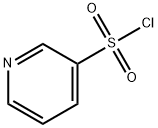
56448-22-7
82 suppliers
inquiry
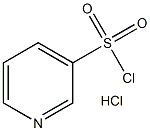
42899-76-3
164 suppliers
$13.43/1gm:

881676-90-0
37 suppliers
inquiry
Yield:881676-90-0 75%
Reaction Conditions:
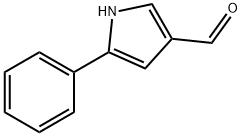
Stage #1: 5-phenyl-1H pyrrole-3-carboxaldehydewith sodium hydride in tetrahydrofuran;mineral oil at 10 - 35; for 0.25 h;Inert atmosphere;
Stage #2: with 15-crown-5 in tetrahydrofuran;mineral oil at 10 - 35; for 0.25 h;Inert atmosphere;
Stage #3: pyridine-3-sulfonyl chloride hydrochloride in tetrahydrofuran;mineral oil at 10 - 35; for 0.5 h;Inert atmosphere;
Steps:
234 5-Phenyl-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde
Reference Example 234
5-Phenyl-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde
Under an argon atmosphere, 5-phenyl-1H-pyrrole-3-carbaldehyde (342 mg) was dissolved in absolute tetrahydrofuran (20 mL) and sodium hydride (60% in oil, 240 mg) was added while stirring at room temperature.
After stirring at the same temperature for 15 min, 15-crown-5 (1.21 mL) was added, and the mixture was further stirred at the same temperature for 15 min. Pyridin-3-ylsulfonyl chloride hydrochloride (642 mg) was added, and the mixture was further stirred at the same temperature for 30 min.
The reaction mixture was diluted with ethyl acetate, washed successively with saturated aqueous sodium hydrogencarbonate solution and saturated brine, and dried over anhydrous magnesium sulfate.
The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate=19:1→1:1) to give the title compound as a brown solid (yield 470 mg, 75%).
1H-NMR (CDCl3)δ: 6.60 (1H, d, J=1.8 Hz), 7.15-7.19 (2H, m), 7.25-7.37 (3H, m), 7.42-7.48 (1H, m), 7.53-7.57 (1H, m), 8.13 (1H, d, J=1.8 Hz), 8.49-8.50 (1H, m), 8.74-8.76 (1H, m), 9.90 (1H, s).
References:
EP2336107,2015,B1 Location in patent:Paragraph 0408

42899-76-3
164 suppliers
$13.43/1gm:

881676-90-0
37 suppliers
inquiry
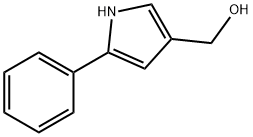
881673-95-6
12 suppliers
inquiry

881676-90-0
37 suppliers
inquiry
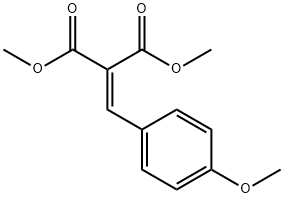
7443-25-6
76 suppliers
$17.00/5G

881676-90-0
37 suppliers
inquiry