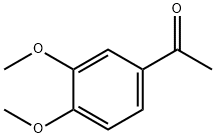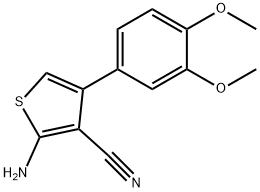
2-AMINO-4-(3,4-DIMETHOXYPHENYL)THIOPHENE-3-CARBONITRILE synthesis
- Product Name:2-AMINO-4-(3,4-DIMETHOXYPHENYL)THIOPHENE-3-CARBONITRILE
- CAS Number:884497-31-8
- Molecular formula:C13H12N2O2S
- Molecular Weight:260.31
Yield:884497-31-8 67%
Reaction Conditions:
Stage #1: malononitrile;1-(3,4-dimethoxyphenyl)ethanonewith ammonium acetate in toluene;Reflux;Green chemistry;Gewald Aminoheterocycles Synthesis;
Stage #2: with sulfur at 60; for 4 h;Ionic liquid;Green chemistry;Gewald Aminoheterocycles Synthesis;
Steps:
4-Aryl-2-aminothiophenes (5a-g) and 4-Alkoxy-2-aminothiophenes(8a-h)
General procedure: A mixture of 2-(1-ethoxyethylidene)malononitrile[27] (7b, 272 mg, 2 mmol),sulfur (64 mg, 2 mmol), and basic ionic liquid (bmIm)OH (380 mg, 2.4 mmol) was allowed to stir at 60°C for 4 h. The reaction mixture was cooled to room temperature and washed with diethyl ether or ethyl acetate (330 mL), and organic layers were concentrated to 1/4 of the volume and kept in a refrigerator. The precipitate that formed was filtered and dried.
References:
Kaki, Venkata Rao;Akkinepalli, Raghuram Rao;Deb, Pran Kishore;Pichika, Mallikarjuna Rao [Synthetic Communications,2015,vol. 45,# 1,p. 119 - 126]