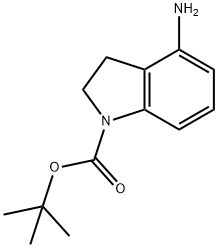
4-AMINO-2,3-DIHYDRO-INDOLE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-AMINO-2,3-DIHYDRO-INDOLE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:885272-42-4
- Molecular formula:C13H18N2O2
- Molecular Weight:234.29
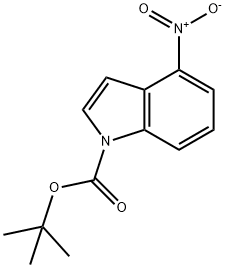
913836-24-5
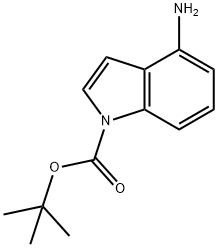
885270-30-4

885272-42-4
The general procedure for the synthesis of tert-butyl 1-Boc-4-aminoindole and 4-amino-2,3-difluoroindole-1-carboxylate from 1-Boc-4-nitroindole is as follows: to a 2.5 liter Paar oscillator hydrogenation flask were added 40.0 grams (0.152 mol) of 1-Boc-4-nitroindole, 500 ml of toluene, and 1.0 gram of 5 wt% Pd/C Degussa Type E101NO/W (50% water wet). The yellow-green mixture is hydrogenated at room temperature for approximately 3 hours at approximately 50 psi hydrogen pressure until hydrogen uptake ceases and HPLC analysis shows that the reaction is complete. Note: Batch analysis should be performed immediately after completion of hydrogen uptake as excessive hydrogenation results in the formation of approximately 5-15% indole by-products (extended reaction times will result in decreased yields due to excessive hydrogenation). The reaction mixture is filtered through Celite, washed with toluene and concentrated. Multiple hydrogenation batches were combined and concentrated to 140 g of crude orange oil. Purification by column chromatography with 25% ethyl acetate: 75% hexane as eluent gives 121 g of pure product as an orange oil with 100% HPLC purity and 88% overall yield. The indole by-product must be removed in this step or it will interfere with subsequent steps.

913836-24-5
61 suppliers
$22.00/250mg

885270-30-4
60 suppliers
$54.00/100 mg

885272-42-4
51 suppliers
$69.50/50 mg
Yield: 88%
Reaction Conditions:
with hydrogen;5% Pd(II)/C(eggshell) in toluene at 20; under 2585.81 Torr; for 3 h;
Steps:
2
To a 2.5 liter Paar shaker hydrogenation flask was charged 40.0 g (0.152 mol) of, 500 mL of toluene and 1.0 g of 5 wt % Pd-C Degussa Type E101 NO/W (50% water wet). The yellow-green mixture was hydrogenated at about 50 psi at room temperature for about 3 hours until no further hydrogen uptake and HPLC analysis showed the reaction to be complete. Note: batch should be analyzed quickly when hydrogen uptake is complete since about 5-15% of indolene by-product forms due to over hydrogenation. (Extended reaction time results in yield loss via over hydrogenation). The mixture was filtered through Celite, washed with toluene, and concentrated.Multiple hydrogenation batches were combined and concentrated to 140 gram crude orange oil. The material was purified by chromatography with 25% EtOAc: 75% hexane and eluted to afford 121 gram of pure product as an orange oil in 100 area % by HPLC in an overall yield of 88%. The indolene by-product 12 must be removed in this step otherwise it carries through to later steps.
References:
Wyeth US2010/36123, 2010, A1 Location in patent:Page/Page column 8

913836-24-5
61 suppliers
$22.00/250mg

885272-42-4
51 suppliers
$69.50/50 mg

24424-99-5
869 suppliers
$13.50/25G

885272-42-4
51 suppliers
$69.50/50 mg
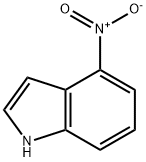
4769-97-5
350 suppliers
$6.00/250mg

885272-42-4
51 suppliers
$69.50/50 mg