
1-[(4-Fluorophenyl)methyl]-5-methyl-1H-1,2,3-triazole-4-carboxylic acid, 4-Carboxy-1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazole synthesis
- Product Name:1-[(4-Fluorophenyl)methyl]-5-methyl-1H-1,2,3-triazole-4-carboxylic acid, 4-Carboxy-1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazole
- CAS Number:885950-27-6
- Molecular formula:C11H10FN3O2
- Molecular Weight:235.21

885950-26-5
17 suppliers
$148.00/500mg
![1-[(4-Fluorophenyl)methyl]-5-methyl-1H-1,2,3-triazole-4-carboxylic acid, 4-Carboxy-1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazole](/CAS/GIF/885950-27-6.gif)
885950-27-6
14 suppliers
$180.00/1g
Yield: 56%
Reaction Conditions:
Stage #1:ethyl 1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazole-4-carboxylate with lithium hydroxide monohydrate in tetrahydrofuran;water at 20; for 16 h;
Stage #2: with hydrogenchloride in water
Steps:
6SI16.3 Synthesis of l-(4-fluorobenzvD-5-methyl-lH-l, 2, 3-triazole-4-carboxylic acid (6S-BL);
[00322] To a stirring solution of 6S-BK-2 (800 mg, 3.04 mmol) in THF/H20 (5 mL/5 mL, 1: 1) were added LiOH.H20 (318 mg, 7.60 mmol) at RT and stirred for 16 h. After completion of reaction (by TLC), the volatiles were evaporated under reduced pressure. The residue was acidified with aqueous 2N HCl and the precipitated solid was filtered and washed with water (5 mL) followed by w-pentane (5 mL) dried under reduced pressure to afford 6S-BL (400 mg, 56%) as white solid.H-NMR(6S-BL): (500 MHz, DMSO-d6): δ 13.02 (br s, 1H), 7.28 (t, J= 8.0 Hz, 2H), 7.20 (t, J = 9.0 Hz, 2H), 5.61 (s, 2H), 2.51 (s, 3H);Mass m/z: 236.1[M++1]
References:
NAUREX, INC.;LOWE, John, A.;KHAN, M., Amin WO2014/120800, 2014, A1 Location in patent:Paragraph 00322; page 132
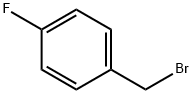
459-46-1
333 suppliers
$10.00/1g
![1-[(4-Fluorophenyl)methyl]-5-methyl-1H-1,2,3-triazole-4-carboxylic acid, 4-Carboxy-1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazole](/CAS/GIF/885950-27-6.gif)
885950-27-6
14 suppliers
$180.00/1g
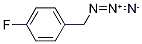
159979-96-1
14 suppliers
$45.00/50mg
![1-[(4-Fluorophenyl)methyl]-5-methyl-1H-1,2,3-triazole-4-carboxylic acid, 4-Carboxy-1-(4-fluorobenzyl)-5-methyl-1H-1,2,3-triazole](/CAS/GIF/885950-27-6.gif)
885950-27-6
14 suppliers
$180.00/1g