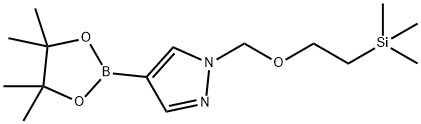
4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole synthesis
- Product Name:4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole
- CAS Number:894807-98-8
- Molecular formula:C15H29BN2O3Si
- Molecular Weight:324.3
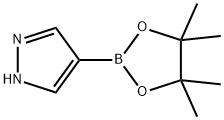
269410-08-4
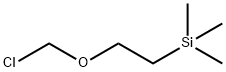
76513-69-4
![4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole](/CAS/20180713/GIF/894807-98-8.gif)
894807-98-8
General procedure: 1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole-4-boronic acid pinacol ester was synthesized from 4-pyrazoleboronic acid pinacol ester and 2-(trimethylsilyl)ethoxymethyl chloride. To a solution of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (8.2 g, 41 mmol) in N-methylpyrrolidone (NMP, 60 mL) was added sequentially potassium carbonate (K2CO3, 12 g, 82 mmol) and 2-(trimethylsilyl)ethoxymethyl chloride (7.8 mL, 43 mmol). The reaction mixture was stirred at room temperature for 16 h under the protection of nitrogen (N2). After completion of the reaction, the mixture was diluted and filtered and the filtrate was diluted with ethyl acetate (EtOAc, 300 mL). The resulting solution was washed sequentially with saturated sodium bicarbonate (NaHCO3) aqueous solution (3×200 mL), water (4×200 mL) and brine (1×200 mL), dried over anhydrous sodium sulfate (Na2SO4), filtered, concentrated and dried in vacuum to afford the intermediate 1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole-4-boronic acid pinacol ester ( 11.4 g, yield 86%) as a light yellow oil.

269410-08-4
394 suppliers
$5.00/1g

76513-69-4
358 suppliers
$6.00/1g
![4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole](/CAS/20180713/GIF/894807-98-8.gif)
894807-98-8
73 suppliers
$98.00/100mg
Yield: 86%
Reaction Conditions:
with potassium carbonate in 1-methyl-pyrrolidin-2-one at 20; for 16 h;Inert atmosphere;
Steps:
15.A
Synthesis of 4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2~yl)-1-((2- (trimethylsilyl)ethoxy)methyl)-1 t-15a)lnt-15aTo a solution of 4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)- H- pyrazole (8.2 g, 41 mmol) in NMP (60 mL) was added K2C03 (12 g, 82 mmoi) and 2-(trimethylsilyi)ethoxymethy. chloride (7.8 mL, 43 mmol) in sequence. The reaction mixture was stirred at r.t. under N2 for 16 h. Then, the reaction mixture was diluted and filtered, and then the filtrate was diluted with EtOAc (300 mL). The resulting solution was washed with sat. NaHC03 (aq) (3 x 200 mL), H20 (4 x 200 mL), brine (1 x 200 mL), dried over Na2S04, filtered, concentrated and dried in vacuo to yield intermediate .nt-15a (11.4 g, 86 %) as a clear yellowish oil.
References:
SCHERING CORPORATION;TSUI, Hon-Chung;PALIWAL, Sunil;KIM, Hyunjin, M.;KEREKES, Angela, D.;CAPLEN, Mary Ann;ESPOSITE, Sara, J.;MCKITTRICK, Brian, A.;FISCHMANN, Thierry Olivier;DOLL, Ronald, J.;RAINKA, Matthew Paul;LI, Ang WO2011/149874, 2011, A2 Location in patent:Page/Page column 81-82

76513-69-4
358 suppliers
$6.00/1g
![4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole](/CAS/20180713/GIF/894807-98-8.gif)
894807-98-8
73 suppliers
$98.00/100mg

73183-34-3
586 suppliers
$6.00/5g
![4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-([2-(trimethylsilyl)ethoxy]methyl)-1H-pyrazole](/CAS/20180713/GIF/894807-98-8.gif)
894807-98-8
73 suppliers
$98.00/100mg