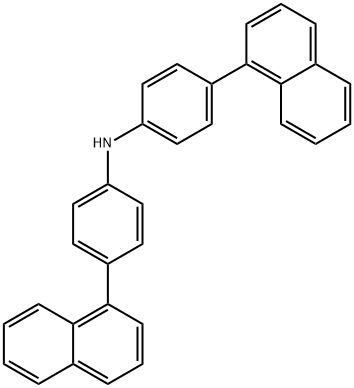
4-(Naphthalenyl)-N-[4-(1-naphthalenyl)phenyl]benzenamine synthesis
- Product Name:4-(Naphthalenyl)-N-[4-(1-naphthalenyl)phenyl]benzenamine
- CAS Number:897671-74-8
- Molecular formula:C32H23N
- Molecular Weight:421.53
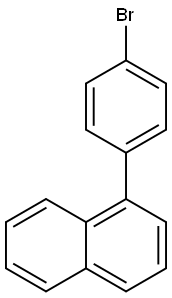
204530-94-9
200 suppliers
$6.00/250mg
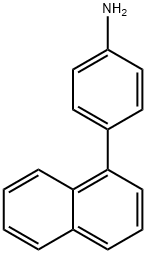
125404-00-4
68 suppliers
inquiry
![4-(Naphthalenyl)-N-[4-(1-naphthalenyl)phenyl]benzenamine](/CAS/20180601/GIF/897671-74-8.gif)
897671-74-8
47 suppliers
inquiry
Yield:897671-74-8 75%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);tri-tert-butyl phosphine;sodium t-butanolate in toluene at 120; for 10 h;Inert atmosphere;
Steps:
3.1 Step 1: Synthesis of Intermediate 7-1
Add 4-(1-naphthyl)aniline (10.4g, 47.4mmol), 1-(4-bromophenyl)naphthalene (13.4g, 47.4mmol), and sodium tert-butoxide (9.1g, 94.8mmol) into the reaction flask in,Replace with nitrogen 3 times, add toluene (100ml) under nitrogen protection, and replace 3 times with nitrogen,Add tris(dibenzylideneacetone) bispalladium (0.43g, 0.474mmol) under nitrogen protection,50% tri-tert-butyl phosphine (0.38g, 0.948mmol), nitrogen replacement 3 times,The temperature was raised to 120°C and stirred for 10h. Cool to room temperature, add 100ml of water and stir for 30min,The solid compound was dried by suction filtration, and 15 g of solid compound was obtained by separation and purification by column chromatography with a yield of 75%.
References:
CN112961134,2021,A Location in patent:Paragraph 0071-0074