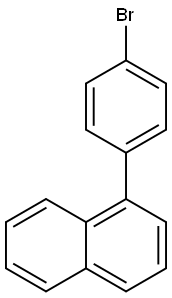
1-(4-Bromophenyl)-naphthlene synthesis
- Product Name:1-(4-Bromophenyl)-naphthlene
- CAS Number:204530-94-9
- Molecular formula:C16H11Br
- Molecular Weight:283.16
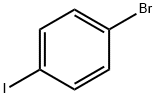
589-87-7
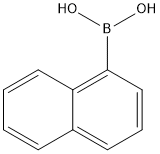
13922-41-3

204530-94-9
Under argon protection, 1-naphthaleneboronic acid (200.0 g, 1.163 mol), 4-bromoiodobenzene (329.0 g, 1.163 mol), tetrakis(triphenylphosphine)palladium(0) (26.9 g, 23.3 mmol), toluene (3.7 L), and 2M aqueous sodium carbonate (1.74 L) were added to the reaction vessel. The reaction mixture was stirred at 90 °C for 24 hours. After completion of the reaction, the mixture was filtered to remove the aqueous phase. The organic phase was washed with water and subsequently dried with magnesium sulfate. The toluene solvent was removed by distillation under reduced pressure. The residue was purified by silica gel column chromatography to afford 1-(4-bromophenyl)naphthalene (268 g, 81% yield).

589-87-7
540 suppliers
$10.00/1g

13922-41-3
493 suppliers
$5.00/1g

204530-94-9
198 suppliers
$6.00/250mg
Yield:204530-94-9 81%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in water;toluene at 90; for 24 h;Inert atmosphere;
Steps:
4.1 [Synthesis Reference 4-1] Synthesis of 1-(4-bromophenyl)naphthalene
Under an argon gas atmosphere, 200.0 g (1.163 mol) of 1-naphthaleneboronic acid, 329.0 g (1.163 mol) of 4-bromoiodobenzene, 26.9 g (23.3 mmol) of tetrakis(triphenylphosphine)palladium(0), 3.7 L of toluene and 1.74 L of aqueous solution of 2M sodium carbonate were added together, and stirred at 90 degrees C. for 24 hours. After the reaction was over, the mixture experienced filtration, through which aqueous phase thereof was eliminated. The organic phase thereof was washed by water and dried with magnesium sulfate, and the toluene was then distilled away under reduced pressure. Residue thereof was refined by silica-gel column chromatography, such that 268 g of 1-(4-bromophenyl) naphthalene was obtained at an yield of 81%.
References:
Idemitsu Kosan Co., Ltd. US2010/331585, 2010, A1 Location in patent:Page/Page column 96
106-37-6
478 suppliers
$9.00/5g

13922-41-3
493 suppliers
$5.00/1g

204530-94-9
198 suppliers
$6.00/250mg
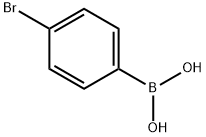
5467-74-3
468 suppliers
$8.00/1g
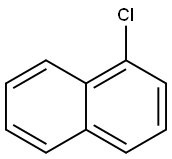
90-13-1
209 suppliers
$17.00/25G

204530-94-9
198 suppliers
$6.00/250mg
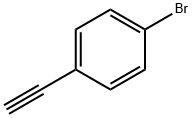
766-96-1
228 suppliers
$7.00/250mg
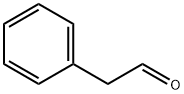
122-78-1
331 suppliers
$10.00/5g

204530-94-9
198 suppliers
$6.00/250mg

589-87-7
540 suppliers
$10.00/1g
33397-22-7
0 suppliers
inquiry

204530-94-9
198 suppliers
$6.00/250mg