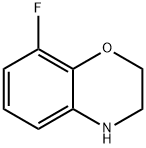
8-FLUORO-3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE HYDROCHLORIDE synthesis
- Product Name:8-FLUORO-3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE HYDROCHLORIDE
- CAS Number:898832-40-1
- Molecular formula:C8H8FNO
- Molecular Weight:153.15
![8-FLUORO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE](/CAS/GIF/560082-51-1.gif)
560082-51-1
![8-FLUORO-3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE HYDROCHLORIDE](/CAS/GIF/898832-40-1.gif)
898832-40-1
8-Fluoro-2H-1,4-benzoxazin-3(4H)-one (1.0 g, 5.9 mmol) was used as starting material, which was dissolved in THF (25 mL) and stirred to form a suspension at 0 °C to -5 °C. Subsequently, a THF solution of lithium aluminum hydride (7 mL, 1 M) was slowly added dropwise. After the dropwise addition, the reaction mixture was warmed to room temperature and stirring was continued for 2 hours. Upon completion of the reaction, the mixture was carefully poured into ice water to quench the reaction and extracted with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to remove the solvent. The resulting residue was ground with ether to afford 8-fluoro-3,4-dihydro-2H-benzo[1,4]oxazine (0.8 g, 87% yield) as a dark brown solid. Mass spectrum (ESI+): m/z 154 [M+H]+. 1H NMR (DMSO-d6): δ 3.27 (2H, m), 4.11 (2H, m), 6.05 (1H, br s), 6.34 (2H, m), 6.58 (1H, m).
![8-FLUORO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE](/CAS/GIF/560082-51-1.gif)
560082-51-1
22 suppliers
inquiry
![8-FLUORO-3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE HYDROCHLORIDE](/CAS/GIF/898832-40-1.gif)
898832-40-1
54 suppliers
$69.00/100mg
Yield:898832-40-1 87%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Steps:
Intermediate 8: 8-Fluoro-3,4-dihydro-2H-benzo[l,4]oxazineTo a stirred mixture of 8-fluoro-2H- l, 4-benzoxazin-3(4H)-one (l .Og, 0.0059mol) in THF (25mL) was added lithium aluminium hydride (7mL, 1M solution in THF) dropwise at 0°C to -5°C. The reaction mixture was stirred at room temperature for 2h and then poured onto ice and extracted with ethyl acetate. The organic phase was dried (MgS04) and the solvent removed under reduced pressure. The residue was triturated with diethyl ether to afford 8-fluoro-3,4-dihydro-2H-benzo[l,4]oxazine (0.8g, 87%) as a dark brown solid.Mass: (ES+) 154 (M+H) + NMR: δΗ ( 6-DMSO) 3.27 (2H, m), 4.11 (2H, m), 6.05 (1H, br s), 6.34 (2H, m) and 6.58 (1H, m).
References:
WO2011/144578,2011,A1 Location in patent:Page/Page column 30
1526-17-6
191 suppliers
$6.00/1g
![8-FLUORO-3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE HYDROCHLORIDE](/CAS/GIF/898832-40-1.gif)
898832-40-1
54 suppliers
$69.00/100mg
698984-51-9
0 suppliers
inquiry
![8-FLUORO-3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE HYDROCHLORIDE](/CAS/GIF/898832-40-1.gif)
898832-40-1
54 suppliers
$69.00/100mg