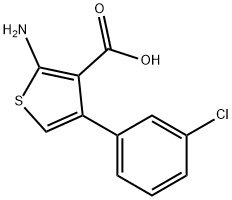
2-AMINO-4-(3-CHLOROPHENYL)THIOPHENE-3-CARBOXYLIC ACID synthesis
- Product Name:2-AMINO-4-(3-CHLOROPHENYL)THIOPHENE-3-CARBOXYLIC ACID
- CAS Number:899691-53-3
- Molecular formula:C11H8ClNO2S
- Molecular Weight:253.7
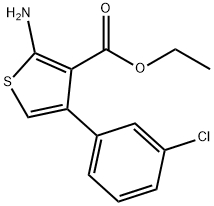
473438-03-8
12 suppliers
inquiry

899691-53-3
5 suppliers
inquiry
Yield:899691-53-3 11 g
Reaction Conditions:
with potassium hydroxide in ethanol; for 20 h;Reflux;
Steps:
5.1.13 N-(4-(3-Chlorophenyl)-5-(3-fluorobenzoyl)thiophen-2-yl)-2-(4-(ethylsulfonyl)phenyl)acetamide (9g)
A mixture of 1-(3-chlorophenyl)ethanone (10c, 30g, 194mmol), ethyl 2-cyanoacetate (66g, 582mmol), sulfur (8g, 252mmol) and morpholine (34g, 388mmol) in ethanol (340mL) was heated to reflux and stirred overnight. Solvent was removed, and the residue was purified by flash chromatography (silica gel, EtOAc:PE=5-10%) to give ethyl 2-amino-4-(3-chlorophenyl)-thiophene-3-carboxylate (17c, 15g, 36% yield) as a yellow solid: MS(ES+) m/z 281.9 (M+H)+. (0033) A solution of KOH (50.8g, 181mmol) was added to a solution of 17c (15.0g, 45.3mmol) in ethanol (200mL). The reaction mixture was heated to reflux for 20h. After cooling to room temperature, solvent was removed. To the residue was added water (150mL), and then the solution was acidified to pH ~7 with 4M HCl, at which point solid precipitated from the solution. The solid was collected by filtration, washed with water, and dried in air to give 2-amino-4-(3-chlorophenyl)thiophene-3-carboxylic acid (11g, 36.9mmol) as a beige solid. Then the solid was dissolved in ethanol (150mL) and 2M HCl (92mL, 184mmol) was added. The reaction mixture was stirred at room temperature for 2h. Solvent was removed in vacuo, and the residue was triturated with diethyl ether to give 4-(3-chlorophenyl)thiophen-2-amine hydrochloride (18c, 7.3g, 56% yield) as a beige solid: MS(ES+) m/z 209.9 (M+H)+. (0034) To a solution of 18c (6.0g, 21.94mmol), 2-(4-(ethylsulfonyl)phenyl)acetic acid 16 (6.5g, 28.5mmol), EDC (6.3g, 32.9mmol) and HOBt (4.4g, 28.5mmol) in dichloromethane (DCM) (90mL) was added dropwise diisopropylethylamine (7.7mL, 43.9mmol) at room temperature. The reaction mixture was heated at reflux under nitrogen overnight. The reaction mixture was partitioned between DCM (150mL) and water (80mL).The organic phase was washed with water (50mL×2), brine (50mL), dried over anhydrous sodium sulfate, and concentrated. The residue was purified by column chromatography (silica gel, EtOAc:PE=25-50%) to give N-(4-(3-chlorophenyl)thiophen-2-yl)-2-(4-(ethylsulfonyl)phenyl)acetamide (19c) (7.0g, 68% yield) as a pale brown solid: MS(ES+) m/z 419.9 (M+H+). (0035) To a solution of 19c (200mg, 0.476mmol) and 3-fluorobenzoyl chloride (151mg, 0.953mmol) in 1,2-dichloroethane (DCE) (16mL) was added dropwise tin(IV) chloride (1M in DCM, 0.953mL, 0.953mmol). The reaction mixture was heated to reflux overnight. The reaction mixture was diluted with DCM (50mL), and then washed with water (20mL). The aqueous phase was extracted with DCM (20mL). The combined organic phase was dried over anhydrous sodium sulfate. After filtration and concentration, the residue was purified by column chromatography (silica gel, EtOAc:PE=1:1 to EtOAc: PE:THF=5:5:2) to give the crude product, which was further purified by MDAP to afford 9g (15mg, 6% yield) as a white solid.
References:
Wang, Yonghui;Cai, Wei;Zhang, Guifeng;Yang, Ting;Liu, Qian;Cheng, Yaobang;Zhou, Ling;Ma, Yingli;Cheng, Ziqiang;Lu, Sijie;Zhao, Yong-Gang;Zhang, Wei;Xiang, Zhijun;Wang, Shuai;Yang, Liuqing;Wu, Qianqian;Orband-Miller, Lisa A.;Xu, Yan;Zhang, Jing;Gao, Ruina;Huxdorf, Melanie;Xiang, Jia-Ning;Zhong, Zhong;Elliott, John D.;Leung, Stewart;Lin, Xichen [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 2,p. 692 - 702]
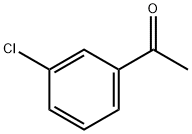
99-02-5
295 suppliers
$9.00/1g

899691-53-3
5 suppliers
inquiry