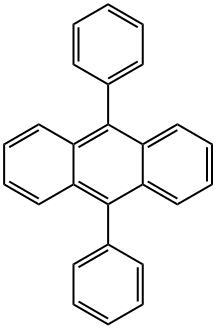
9,10-Diphenylanthracene synthesis
- Product Name:9,10-Diphenylanthracene
- CAS Number:1499-10-1
- Molecular formula:C26H18
- Molecular Weight:330.42
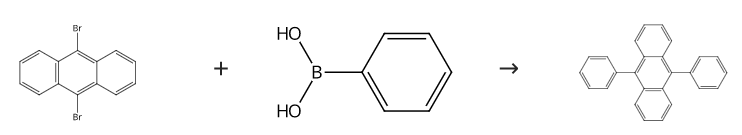
The mixture of 1,3-dibromobenzene (236 mg, 1.0 mmol), phenylboronic acid (271 mg, 2.2 mmol), and Na2CO3 (424 mg, 4 mmol) was taken up in DMF (2 mL) and then ARF-Pd (300 mg, 0.01 mmol Pd) was added. The mixture was heated under N2 at 110 °C for 2.5 h. After cooling, the mixture was diluted with cold H2O (10 mL) and filtered through a cotton bed. The filtrate was extracted with Et2O (3 × 20 mL) and the combined organic extracts were washed with brine (10 mL), dried (Na2SO4), and evaporated. The residue was purified by column chromatography (silica gel, PE) to give 9,10-Diphenylanthracene (4d), yield 90%. Mp 245-247 °C (Lit.36 245-48 °C). IR (Nujol): 1458, 1377 cm-1. 1H NMR (300 MHz, CDCl3): δ = 7.72-7.66 (m, 4 H), 7.62-7.46 (m, 10 H), 7.34-7.29 (m, 4 H). 13C NMR (75 MHz, CDCl3): δ = 139.1, 137.1, 131.3, 129.8, 128.4, 127.4, 126.9, 125.0.
19061-38-2
0 suppliers
inquiry

1499-10-1
322 suppliers
$10.00/1g
Yield:1499-10-1 94%
Reaction Conditions:
with triethylsilane;trifluoroacetic acid in dichloromethane at 20; for 0.5 h;Inert atmosphere;Sealed tube;
Steps:
9,10-Diphenylanthracene (4a)
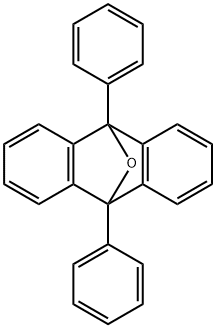
A 10-mL test tube equipped with a magnetic stirring bar and a cap was charged with9,10-diphenyl-9,10-epoxyanthracene (3a) (102 mg, 295 μmol), wet dichloromethane (600μL), triethylsilane (71.9 μL, 450 μmol), and trifluoroacetic acid (26.7 μL, 360 μmol). Themixture was stirred for 30 min, after which time TLC (hexanes-ethyl acetate = 3:1) indicatedcomplete consumption of 9,10-diphenyl-9,10-epoxyanthracene (3a). The reaction wasquenched with H2O and the aqueous layer was extracted with dichloromethane three times.The combined organic extracts were successively washed with saturated aqueous NaHCO3and brine, dried over anhydrous Na2SO4, and filtered. The organic solvents were removedunder reduced pressure to give a crude anthracene, which was purified by flash silica gelcolumn chromatography (hexanes-dichloromethane = 4:1) to afford 4a (91.4 mg, 277 μmol,94%) as a pale yellow solid. Rf = 0.73 (hexanes-ethyl acetate = 3:1). The spectrum data of 4awere in complete agreement with those reported in the literature.5
References:
Miyamoto, Naoya;Nakazawa, Yuki;Nakamura, Takanori;Okano, Kentaro;Sato, Sota;Sun, Zhe;Isobe, Hiroyuki;Tokuyama, Hidetoshi [Synlett,2018,vol. 29,# 4,art. no. ST-2017-U0659-L,p. 513 - 518] Location in patent:supporting information
6318-17-8
6 suppliers
$28.60/25mg

1499-10-1
322 suppliers
$10.00/1g
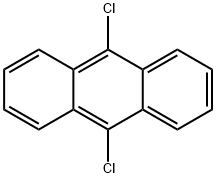
605-48-1
113 suppliers
$29.00/1g
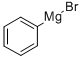
100-58-3
196 suppliers
$18.00/10ml

1499-10-1
322 suppliers
$10.00/1g
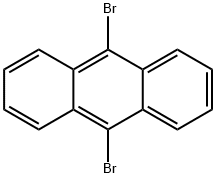
523-27-3
392 suppliers
$6.00/5g
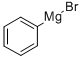
100-58-3
196 suppliers
$18.00/10ml

1499-10-1
322 suppliers
$10.00/1g
