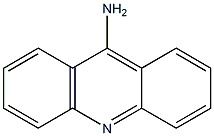
9-ACETAMIDOACRIDINE synthesis
- Product Name:9-ACETAMIDOACRIDINE
- CAS Number:23043-52-9
- Molecular formula:
- Molecular Weight:0
Yield:23043-52-9 73%
Reaction Conditions:
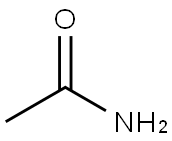
Stage #1: acetamidewith sodium hydride in dimethyl sulfoxide;paraffin oil; for 0.5 h;
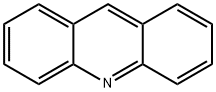
Stage #2: acridinewith potassium hexacyanoferrate(III) in dimethyl sulfoxide;paraffin oil at 20; for 10 h;
Steps:
Synthesis of N-(acridin-9-yl)acylamides 2-10 (Generalmethod).
General procedure: The reaction was performed in a vessel protectedfrom air moisture. Sodium hydride (60% suspension in oil,120 mg, 3 mmol) was added with stirring to a solution ofthe appropriate amide (3 mmol) in anhydrous DMSO(4 ml). After the evolution of hydrogen ceased (~0.5 h), thereaction mixture was treated with acridine (1) (89.5 mg,0.5 mmol), K3Fe(CN)6 (1 g, 3 mmol) and vigorously stirredat room temperature for the duration indicated in Table 1.Then the mixture was poured into cold water (50 ml) andacidified with dilute HCl solution to pH ~7. The precipitatewas filtered off, washed with water, dried (when isolatingthe amide 5, the precipitate on filter was washed at firstwith 100 ml of hot water (~90°C) to remove the exess of 4-nitrobenzamide). The products were further purified byrecrystallization from the appropriate solvents.
References:
Demidov, Oleg P.;Borovlev, Ivan V.;Amangasieva, Gulminat A.;Avakyan, Elena K. [Chemistry of Heterocyclic Compounds,2016,vol. 52,# 2,p. 104 - 109][Khim. Geterotsikl. Soedin.,2016,vol. 52,# 2,p. 104 - 109,6]