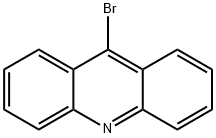
9-BroMoacridine synthesis
- Product Name:9-BroMoacridine
- CAS Number:4357-57-7
- Molecular formula:C13H8BrN
- Molecular Weight:258.11
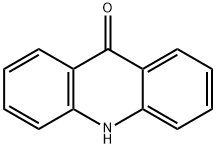
578-95-0
312 suppliers
$5.00/1g

4357-57-7
25 suppliers
$129.00/1g
Yield: 72%
Reaction Conditions:
with phosphorus tribromide at 0 - 100; for 24 h;Inert atmosphere;
Steps:
5.2.6 9-Bromoacridine (7)
PBr3 (19.5mL, 205mmol) was added dropwise to a 250mL round bottom flask containing 3 (4.0g, 20.5mmol) at 0°C under N2 atmosphere. After stirring at 110°C for 24h, the resultant solution was quenched by slow addition of H2O and an aqueous NaOH solution was added to the resultant mixture until pH=14. The crude product was extracted with CH2Cl2 (50mL×3). The combined organic extracts were washed with brine, dried over Na2SO4, filtrated, and concentrated under reduced pressure to obtain the title compound as a yellow solid (3.81g, 72%). 1H NMR (400MHz, Chloroform-d) δ 8.45 (d, J=8.8Hz, 2H), 8.27 (d, J=8.6Hz, 2H), 7.85-7.81 (m, 2H), 7.69-7.65 (m, 2H). MS (ESI) m/z calcd for C13H8BrN [M+ H]+, 258.0, found: 260.2.
References:
Song, Di;Zhang, Nan;Zhang, Panpan;Zhang, Na;Chen, Weijin;Zhang, Long;Guo, Ting;Gu, Xiaotong;Ma, Shutao [European Journal of Medicinal Chemistry,2021,vol. 221,art. no. 113480]
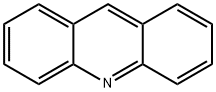
260-94-6
172 suppliers
$20.00/1g

4357-57-7
25 suppliers
$129.00/1g
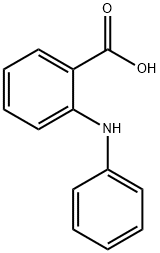
91-40-7
333 suppliers
$10.00/1g

4357-57-7
25 suppliers
$129.00/1g
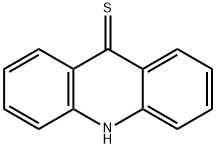
6540-78-9
2 suppliers
inquiry

4357-57-7
25 suppliers
$129.00/1g

62-53-3
688 suppliers
$10.00/1g

4357-57-7
25 suppliers
$129.00/1g