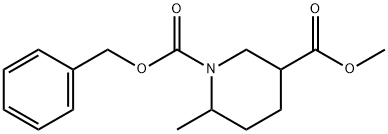
1-benzyl 3-Methyl 6-Methylpiperidine-1,3-dicarboxylate synthesis
- Product Name:1-benzyl 3-Methyl 6-Methylpiperidine-1,3-dicarboxylate
- CAS Number:908245-08-9
- Molecular formula:C16H21NO4
- Molecular Weight:291.34
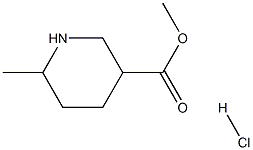
1009376-74-2
18 suppliers
$45.00/2.5mg

501-53-1
394 suppliers
$10.00/1g

908245-08-9
5 suppliers
$615.00/5g
Yield:908245-08-9 18.8%
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 5 - 20; pH=8 - 9; for 2.5 h;
Steps:
B
A 250-mL, four-neck, round-bottomed flask, fitted with a magnetic stirrer was charged with 6-Me-2 (13.3 g, 68.4 mmol), tetrahydrofuran (60 mL), water (60 mL) and sodium bicarbonate (14.4 g, 171 mmol). The reaction mixture was cooled to 5°C. While keeping the pH between pH 8 to pH 9, benzylchloroforniate (12.0 g, 70.4 mmol) was added slowly over 90 min. The mixture was stirred at ambient temperature for 1 h. The reaction mixture was placed under reduced pressure to remove most of the tetrahydrofuran. Ethyl acetate (50 mL) was then added and the mixture was stirred for 5 min. The layers were separated and the organic layer was washed with water (20 mL). The ethyl acetate layer was dried over anhydrous magnesium sulfate. This mixture was filtered and the filtrate was evaporated under reduced pressure to give crude product (16.5 g). The crude product was split in two equal portions. One portion was placed on a 40 mm diameter flash column, packed with silica gel (225 g) using 230:30:3 CHCl3/EtOAc/MeOH. The column was eluted with 230:30:3 CHC I3ZEtO Ac/MeOH. The fractions containing the purest product were combined and the solvents evaporated to dryness under EPO
References:
WO2006/91697,2006,A1 Location in patent:Page/Page column 33-34