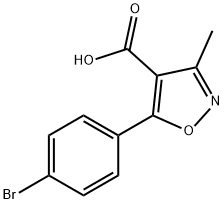
5-(4-Bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid synthesis
- Product Name:5-(4-Bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid
- CAS Number:91182-60-4
- Molecular formula:C11H8BrNO3
- Molecular Weight:282.09
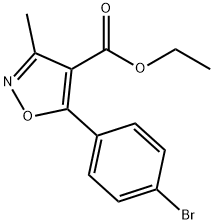
917388-58-0

91182-60-4
General procedure for the synthesis of 5-(4-bromophenyl)-3-methyl-isoxazole-4-carboxylic acid from ethyl 5-(4-bromophenyl)-3-methyl-isoxazole-4-carboxylate: to a stirred THF/H2O (79/35 ml) solution of ethyl 5-(4-bromophenyl)-3-methyl-isoxazole-4-carboxylate (3.2 g, 0.010 mol) was added lithium hydroxide ( 2.4 g, 0.010 mol). The reaction mixture was stirred at room temperature overnight and the THF solvent was removed from the reaction mixture under reduced pressure. The residue was diluted with water and washed with ether (2 x 50 ml). Subsequently, the aqueous phase was acidified to pH 4 with saturated aqueous NaHCO3 and extracted with ethyl acetate (2 x 75 ml). The organic layers were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the pure product 5-(4-bromophenyl)-3-methyl-isoxazole-4-carboxylic acid 2.8 g (96% yield). Product characterization data: 1H NMR (DMSO-d6): δ 2.52 (3H, s), 7.37 (2H, dd), 7.91 (2H, dd), 12.42 (1H, br s). 13C NMR (DMSO-d6) δ 12.0,110.0,126.6,127.2,131.7,132.5,162.4. 164.4,172.7. mass spectrum (MH+) = 282.

917388-58-0
6 suppliers
inquiry

91182-60-4
69 suppliers
$37.00/100mg
Yield:91182-60-4 96%
Reaction Conditions:
Stage #1: 5-(4-bromophenyl)-3-methylisoxazole-4-carboxylic acid ethyl esterwith lithium hydroxide;water in tetrahydrofuran at 20;
Stage #2: with oxalic acid in water; pH=~ 4;
Steps:
AH.4
To a stirred solution of ester (3.2 g, 0.010M) in THF/H2O (79/35 ml) was added lithium hydroxide (2.4 g, 0.010M). The reaction mixture was stirred at room temperature for over night and the solvent, THF was removed from the reaction mixture under vacuum. The resulting mass was diluted with water and washed with diethyl ether (2×50 ml). Then the aqueous solution was acidified with sat. Oxalic acid (PH4) and was extracted with ethyl acetate (2×75 ml). The combined organic layer was dried over anhydrous sodium sulphate, concentrated under vacuum to give the pure product 2.8 g (96%). 1H NMR (DMSO-d6): δ 2.52 (3H, s), 7.37 (2H, dd), 7.91 (2H, dd), 12.42 (1H, br s). 13C NMR (DMSO-d6) δ 12.0, 110.0, 126.6, 127.2, 131.7, 132.5, 162.4, 164.4, 172.7. MH+=282.
References:
US2006/287287,2006,A1 Location in patent:Page/Page column 31-32
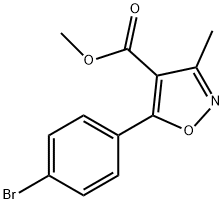
1228689-61-9
30 suppliers
$22.00/100mg

91182-60-4
69 suppliers
$37.00/100mg
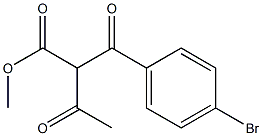
85963-90-2
0 suppliers
inquiry

91182-60-4
69 suppliers
$37.00/100mg
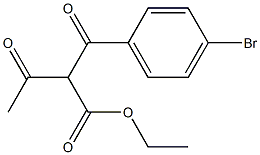
893643-73-7
3 suppliers
inquiry

91182-60-4
69 suppliers
$37.00/100mg
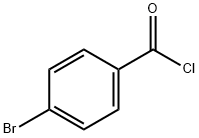
586-75-4
227 suppliers
$10.00/5g

91182-60-4
69 suppliers
$37.00/100mg