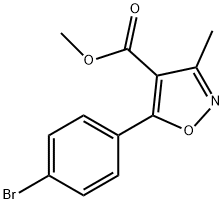
4-Isoxazolecarboxylic acid, 5-(4-broMophenyl)-3-Methyl-, Methyl ester synthesis
- Product Name:4-Isoxazolecarboxylic acid, 5-(4-broMophenyl)-3-Methyl-, Methyl ester
- CAS Number:1228689-61-9
- Molecular formula:C12H10BrNO3
- Molecular Weight:296.12
![Benzenepropanoic acid, 4-bromo-α-[1-(methylamino)ethylidene]-β-oxo-, methyl ester](/CAS/20180713/GIF/1423699-80-2.gif)
1423699-80-2

1228689-61-9
To a stirred solution of methyl 2-(4-bromobenzoyl)-3-(methylamino)but-2-enoate (0.78 g, 2.5 mmol) in acetic acid (10 mL) was added hydroxylamine hydrochloride (0.17 g, 2.5 mmol) under nitrogen atmosphere. After addition, the reaction mixture was heated to reflux for 2 hours under nitrogen protection. Upon completion of the reaction, the mixture was concentrated under reduced pressure to afford the crude product methyl 5-(4-bromophenyl)-3-methylisoxazole-4-carboxylate (0.66 g, 89% yield), which could be used for subsequent reactions without further purification.
![Benzenepropanoic acid, 4-bromo-α-[1-(methylamino)ethylidene]-β-oxo-, methyl ester](/CAS/20180713/GIF/1423699-80-2.gif)
1423699-80-2
14 suppliers
$28.00/100mg

1228689-61-9
30 suppliers
$22.00/100mg
Yield:1228689-61-9 89%
Reaction Conditions:
with hydroxylamine hydrochloride;acetic acid for 2 h;Inert atmosphere;Reflux;
Steps:
I-A
To a stirred solution of I-4a (0.78 g, 2.5 mmol) in HO Ac (10 mL) was added hydroxylamine hydrochloride (0.17 g, 2.5 mmol) under nitrogen. After the addition, the solution was heated to reflux under nitrogen for 2 hrs. The solution was concentrated in vacuo to afford compound I-5a (0.66 g, yield 89%), which was used for next step without further purification.
References:
WO2013/25733,2013,A1 Location in patent:Paragraph 0404
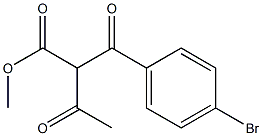
85963-90-2
0 suppliers
inquiry

1228689-61-9
30 suppliers
$22.00/100mg
105-45-3
694 suppliers
$5.00/5g

1228689-61-9
30 suppliers
$22.00/100mg
586-76-5
505 suppliers
$10.00/1g

1228689-61-9
30 suppliers
$22.00/100mg
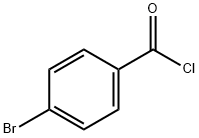
586-75-4
227 suppliers
$10.00/5g

1228689-61-9
30 suppliers
$22.00/100mg