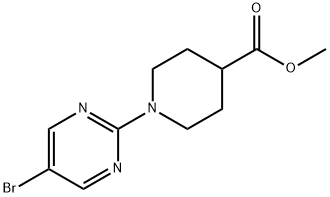
METHYL 1-(5-BROMOPYRIMIDIN-2-YL)PIPERIDINE-4-CARBOXYLATE synthesis
- Product Name:METHYL 1-(5-BROMOPYRIMIDIN-2-YL)PIPERIDINE-4-CARBOXYLATE
- CAS Number:914347-01-6
- Molecular formula:C11H14BrN3O2
- Molecular Weight:300.15
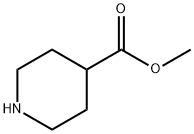
2971-79-1
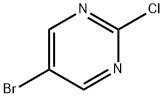
32779-36-5

914347-01-6
Step 1: DIPEA (4 mL, 23.20 mmol) was added dropwise to a solution of acetonitrile (80 mL) containing 5-bromo-2-chloropyrimidine (3.0 g, 15.50 mmol) under nitrogen protection. Subsequently, methyl 4-piperidinecarboxylate (3.321 g, 23.20 mmol) was added to the reaction system. The reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, the solvent was concentrated to dryness by rotary evaporator. Deionized water and ethyl acetate (EtOAc) were added to quench the reaction and the organic layer was separated. The organic phase was dried over anhydrous sodium sulfate and concentrated again to dryness. The crude product was purified by silica gel column chromatography with an elution gradient of heptane/ethyl acetate (100:0 to 0:100). The fractions containing the target product were collected, combined and concentrated to dryness to give methyl 1-(5-bromopyrimidin-2-yl)piperidine-4-carboxylate (1.84 g, 41% yield) as a white solid.

2971-79-1
270 suppliers
$10.00/10g

32779-36-5
631 suppliers
$5.00/5g

914347-01-6
46 suppliers
$22.00/250mg
Yield:914347-01-6 41%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile at 20; for 16 h;
Steps:
1a.1 methyl 1-(5-bromopyrimidin-2-yl)piperidine-4-carboxylate
Step 1 : DIPEA (4 mL, 23.20 mmol) was added to 5-bromo-2-chloropyrimidine (3.0 g, 15.50 mmol) in acetonitrile (80 mL). Then, methyl isonipecotate (3.321 g, 23.20 mmol) was added to the solution. The reaction mixture was stirred at rt for Ex.9a 16h. The solvent was concentrated to dryness. Water and EtOAc were added to quench the reaction. The organic layer was separated and concentrated to dryness. The crude material was purified by silica gel column chromatography eluting with heptane and a gradient of heptane/EtOAc from [100:0] to [0:100]. The product fractions were combined and concentrated to dryness to afford methyl 1-(5-bromopyrimidin-2-yl)piperidine-4-carboxylate Ex.9a (1 .84 mg, 41 %) as white solid.
References:
WO2018/138356,2018,A1 Location in patent:Page/Page column 35; 40-41