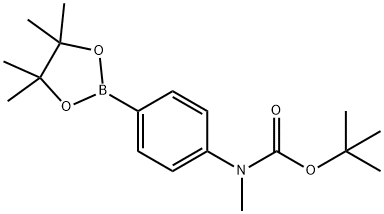
Methyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-carbamic acid tert-butyl ester synthesis
- Product Name:Methyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-carbamic acid tert-butyl ester
- CAS Number:916587-44-5
- Molecular formula:C18H28BNO4
- Molecular Weight:333.23
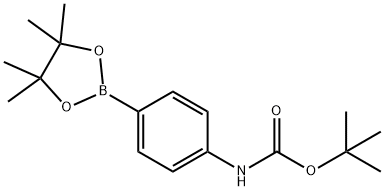
330793-01-6
181 suppliers
$7.00/250mg

74-88-4
356 suppliers
$15.00/10g
![Methyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-carbamic acid tert-butyl ester](/CAS/GIF/916587-44-5.gif)
916587-44-5
42 suppliers
$40.00/250mg
Yield:916587-44-5 70%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 20; for 18 h;
Steps:
2.1 Example 2 N-hydroxy-N'-methyl-2-(methyl{[4'-(methylamino)biphenyl-4-yl]carbonyl}amino)propanediamide (Compound 2)
(1) 60% Sodium hydride (0.55 g) and methyl iodide (1.2 mL) were added to a DMF (6.0 mL) solution of t-butyl(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)carbamate (2.0 g), and the mixture was stirred for 18 hours at room temperature. Ethyl acetate and water were added to the reaction mixture, and the organic layer was isolated. The extract was washed sequentially with water and brine, and dried over anhydrous sodium sulfate. Then, the desiccant was filtered out, whereafter the solvent was distilled off under reduced pressure. Hexane was added to the residue, and the precipitated solid was collected by filtration to obtain t-butyl=methyl(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)carbamate (white solid) (1.46 g, 70%).1 H NMR (400 MHz, CHLOROFORM-d) δ ppm 1.34 (12H, s), 1.45 (9H, s), 3.27 (3H, s), 7.24 (2H, d, J=8.4 Hz), 7.76 (2H, d, J=8.4 Hz)
References:
EP2562155,2013,A1 Location in patent:Paragraph 0222-0223

73183-34-3
586 suppliers
$6.00/5g
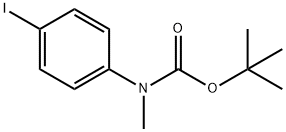
306768-12-7
9 suppliers
inquiry
![Methyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-carbamic acid tert-butyl ester](/CAS/GIF/916587-44-5.gif)
916587-44-5
42 suppliers
$40.00/250mg

24424-99-5
869 suppliers
$13.50/25G
![Methyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-carbamic acid tert-butyl ester](/CAS/GIF/916587-44-5.gif)
916587-44-5
42 suppliers
$40.00/250mg
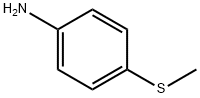
104-96-1
191 suppliers
$10.00/1g
![Methyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-carbamic acid tert-butyl ester](/CAS/GIF/916587-44-5.gif)
916587-44-5
42 suppliers
$40.00/250mg
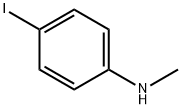
60577-34-6
82 suppliers
$73.32/0.5g
![Methyl-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-carbamic acid tert-butyl ester](/CAS/GIF/916587-44-5.gif)
916587-44-5
42 suppliers
$40.00/250mg