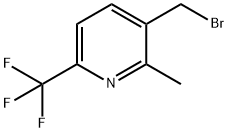
3-(Bromomethyl)-2-methyl-6-(trifluoromethyl)pyridine synthesis
- Product Name:3-(Bromomethyl)-2-methyl-6-(trifluoromethyl)pyridine
- CAS Number:917396-30-6
- Molecular formula:C8H7BrF3N
- Molecular Weight:254.05
![[2-Methyl-6-(trifluoromethyl)pyridin-3-yl]methanol](/CAS/GIF/681260-50-4.gif)
681260-50-4
36 suppliers
$45.00/50mg

917396-30-6
17 suppliers
$99.00/250mg
Yield:917396-30-6 71%
Reaction Conditions:
with hydrogen bromide in water at 135; for 24 h;
Steps:
Preparation of Reagent 3-(bromomethyl)-2-methyl-6-(trifluoromethyl)pyridine
Preparation of Reagent 3-(bromomethyl)-2-methyl-6-(trifluoromethyl)pyridine A mixture of (2-methyl-6-(trifluoromethyl)pyridin-3-yl)methanol (1.677 g, 8.77 mmol, 76% pure) in 48% aq. HBr (10 mL) was heated to reflux (oil bath temperature, 135° C.) for 24 h. The volatile components of the resulting biphasic mixture were distilled under vacuum to remove most of the hydrogen bromide (sodium hydroxide trap). The residue was partitioned between 2:1 EtOAc/THF and water (at pH 3) and the organic phase was dried (MgSO4), filtered and evaporated. The solid residue was re-evaporated after addition of dichloromethane/hexanes to obtain the title compound as a tan solid, 1.19 g, 71% yield (based on the purity of the starting material). HPLC/MS: retention time=2.15 min, [M+H]30 =254.
References:
US2006/287341,2006,A1 Location in patent:Page/Page column 43
205582-88-3
41 suppliers
$26.00/250mg

917396-30-6
17 suppliers
$99.00/250mg