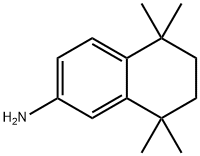
5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-ylamine synthesis
- Product Name:5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-ylamine
- CAS Number:92050-16-3
- Molecular formula:C14H21N
- Molecular Weight:203.32
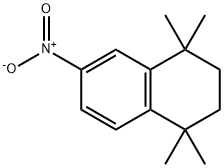
102121-55-1

92050-16-3
General procedure for the synthesis of 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-amine (74) from 2-nitro-5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-amine (73): 1,1,4,4-tetramethyl-6-nitro-1,2,3,4-tetrahydronaphthalene (73) (2.5 g, 10.7 mmol) was dissolved in ethyl acetate (210 mL) in ethyl acetate using 10% Pd/C as a catalyst, and the hydrogenation reaction was repeated twice at a flow rate of 1.0 mL/min in a ThalesNano H-Cube?reactor at 70°C and 2-5 bar hydrogen pressure. Upon completion of the reaction, the reaction solution was concentrated in vacuum to afford a yellow crystalline solid 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-amine (74) (2.1532 g, 99% yield), with a melting point of 205°C. The product was characterized as follows: 1H NMR (400 MHz, CDCl3) δ 7.11 (d, J = 8.4 Hz, 1H), 6.65 (d, J = 2.4 Hz, 1H), 6.54 (dd, J = 8.4, 2.4 Hz, 1H), 3.62 (br s, 2H), 1.66 (s, 4H), 1.26 (s, 6H), 1.25 (s, 6H); 13C NMR (100.6 MHz, CDCl3) δ 145.8, 143.3, 135.4, 127.3, 113.7, 112.9, 35.2, 34.1, 33.5, 31.9, 31.7; IR (neat) 3405, 3208, 2952, 2920, 1612, 1499 cm-1; LC-MS (CI) m/z (M+H)+ Calculated C14H22N 204.1752, Measured C14H22N 204.1752. 204.1747.

102121-55-1
23 suppliers
inquiry

92050-16-3
100 suppliers
$44.00/100mg
Yield:92050-16-3 99%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethyl acetate at 70; under 1500.15 - 3750.38 Torr;
Steps:
7.a a. 5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-amine (74)
a. 5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-amine (74). A 0.05 M solution of1,1 ,4,4-tetramethyl-6-nitro- 1,2,3 ,4-tetrahydronaphthalene (73) (2.5 g, 10.7 mmol) in ethyl acetate(210 mL) was passed through a 10% PdJC cartridge at 1.0 mL/minute, twice, in the ThalesNano Hcube at 70 °C and 2-5 bar pressure. The resulting solution was concentrated in vacuo to give 74(2.1532 g, 99%) as a yellow, crystalline solid, m.p. 58-60 °C: ‘H NMR (400 MHz, CDC13) 7.11 (d, J= 8.4, 1H), 6.65 (d, J= 2.4, 1H), 6.54 (dd, J= 8.4, 2.4, ill), 3.62 (br s, 2H), 1.66 (s, 4H), 1.26 (s, 611), 1.25 (s, 611); ‘3C NMR (100.6 MHz, CDC13) ? 145.8, 143.3, 135.4, 127.3, 113.7, 112.9, 35.2,34.1, 33.5, 31.9, 31.7; IR (neat) 3405, 3208, 2952, 2920, 1612, 1499 cm’; LC-MS-CI (M+H)+ calcd for C,4H22N 204.1752, found 204.1747.
References:
WO2015/130973,2015,A1 Location in patent:Page/Page column 34; 35
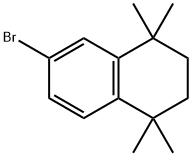
27452-17-1
163 suppliers
$11.00/250mg

92050-16-3
100 suppliers
$44.00/100mg
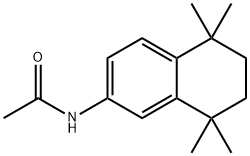
139162-43-9
23 suppliers
inquiry

92050-16-3
100 suppliers
$44.00/100mg
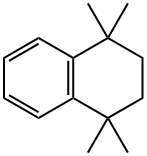
6683-46-1
121 suppliers
$9.00/100mg

92050-16-3
100 suppliers
$44.00/100mg
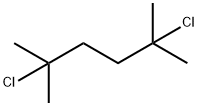
6223-78-5
134 suppliers
$9.00/5g

92050-16-3
100 suppliers
$44.00/100mg