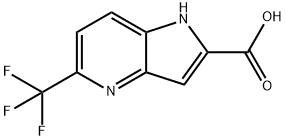
5-(trifluoromethyl)-1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid synthesis
- Product Name:5-(trifluoromethyl)-1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid
- CAS Number:920979-05-1
- Molecular formula:C9H5F3N2O2
- Molecular Weight:230.14
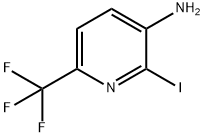
920979-04-0
63 suppliers
$47.00/100mg
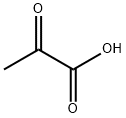
127-17-3
566 suppliers
$5.00/10g
![5-(trifluoromethyl)-1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid](/CAS/20150408/GIF/920979-05-1.gif)
920979-05-1
34 suppliers
inquiry
Yield:-
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane in N,N-dimethyl-formamide at 130; for 4 h;Heating / reflux;
Steps:
19.2
19.2 5-trifluoromethylpyrrolo[3,2-b]pyridine-2-carboxylic acid; 0.5 g (1.74 mmol) of 3-amino-2-iodo-6-trifluoromethylpyridine, obtained in step 19.1, 0.45 g (5.21 mmol) of pyruvic acid, 0.51 ml (5.21 mmol) of 1,4-diazabicyclo[2.2.2]octane and 10 ml of dry dimethylformamide are placed in a sealed tube under argon. The solution is degassed for a few minutes, 0.19 g (0.87 mmol) of palladium acetate is then added and the tube is closed and refluxed for 4 hours at 130° C. The cooled solution is then concentrated under reduced pressure and the resulting residue is taken up in 100 ml of ethyl acetate. The organic phase is successively washed with twice 50 ml of aqueous 2N sodium hydroxide solution. The basic aqueous phases are combined, cooled to 0° C., acidified by addition of hydrochloric acid and then extracted with 4 times 50 ml of ethyl acetate. These organic phases are combined, washed with 20 ml of saturated aqueous sodium chloride solution, dried over sodium sulfate and then concentrated under reduced pressure. 0.22 g of product is obtained, and is used without further purification in the following step.
References:
US2008/125459,2008,A1 Location in patent:Page/Page column 16
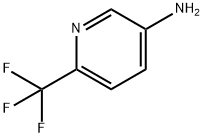
106877-33-2
268 suppliers
$8.00/1g
![5-(trifluoromethyl)-1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid](/CAS/20150408/GIF/920979-05-1.gif)
920979-05-1
34 suppliers
inquiry