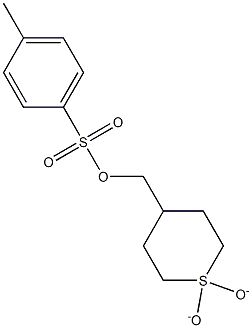
(1,1-dioxidotetrahydro-2H-thiopyran-4-yl)methyl 4-methylbenzenesulfonate synthesis
- Product Name:(1,1-dioxidotetrahydro-2H-thiopyran-4-yl)methyl 4-methylbenzenesulfonate
- CAS Number:928149-12-6
- Molecular formula:C13H18O5S2
- Molecular Weight:318.41
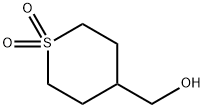
473254-28-3
31 suppliers
$45.00/10mg

98-59-9
626 suppliers
$9.00/5g

928149-12-6
30 suppliers
$578.00/1g
Yield:928149-12-6 99%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 10; for 3 h;
Steps:
95.1 Step 1 : Synthesis of 1 , 1 -dioxidotetrahydro-2H-thiopyran-4-yl)methyl 4- methylbenzenesulfonate
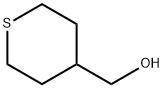
4-Toluenesulfonyl chloride (52.2 g, 274 mmol) was added to a solution of 4- (hydroxymethyl)tetrahydro-2H-thiopyran 1 ,1 -dioxide (compound of step 1 , 30 g, 183 mmol) followed by the addition of triethylamine (76 ml, 548 mmol) and DMAP (2.232 g, 18.27 mmol) in DCM ( 300 ml) at 10 °C and the reaction mixture was stirred for 3 h. After completion of reaction, the reaction mixture was diluted with DCM (300 ml) and washed with water twice (500 ml). The organic layer was dried over sodium sulfate and concentrated. The residue was dissolved in 10% ethyl acetate/petroleum ether (180 ml) and stirred for 2 h. The solid was filtered and washed with 10% ethyl acetate /petroleum ether (100 ml) to obtain the title compound (57.50 g, 181 mmol). Yield: 99 %; 1 H NMR (CDCI3, 300 MHz): δ 7,80 (d, J = 8.1 Hz, 2H), 7.39 (d, J = 8.1 Hz, 2H), 3.91 (d, J = 6.0 Hz, 2H), 3.087-2.912 (m, 4H), 2.482 (s, 3H), 2.151 (d, J = 13.8 Hz, 2H), 2.022-1 .794 (m, 3H); MS: (m/z) 341 .1 (M+Na).
References:
WO2014/170842,2014,A2 Location in patent:Page/Page column 166
100277-27-8
58 suppliers
$50.00/250mg

928149-12-6
30 suppliers
$578.00/1g

473254-28-3
31 suppliers
$45.00/10mg

928149-12-6
30 suppliers
$578.00/1g
1072-72-6
279 suppliers
$10.00/1g

928149-12-6
30 suppliers
$578.00/1g
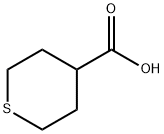
89489-53-2
88 suppliers
$32.00/1g

928149-12-6
30 suppliers
$578.00/1g