
1H-Pyrrolo[2,3-b]pyridine, 5-nitro-1-(phenylsulfonyl)- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine, 5-nitro-1-(phenylsulfonyl)-
- CAS Number:937012-11-8
- Molecular formula:C13H9N3O4S
- Molecular Weight:303.29
![5-NITRO-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/101083-92-5.gif)
101083-92-5
161 suppliers
$12.00/100mg
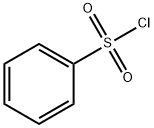
98-09-9
364 suppliers
$12.00/25g
![1H-Pyrrolo[2,3-b]pyridine, 5-nitro-1-(phenylsulfonyl)-](/CAS/GIF/937012-11-8.gif)
937012-11-8
21 suppliers
inquiry
Yield:937012-11-8 95%
Reaction Conditions:
Stage #1: 5-nitro-1H-pyrrolo[2,3-b]pyridinewith potassium tert-butylate in tetrahydrofuran at 20; for 0.5 h;
Stage #2: benzenesulfonyl chloride in tetrahydrofuran at 20; for 2 h;
Steps:
1.1; 5.1
Step 1: Potassium tert-butoxide (720.72 mg, 6.435 mmol, 1.5 equivalents) was added to a solution of compound 5-1 (700 mg, 4.29 mmol, 1.0 equivalent) in tetrahydrofuran (10 mL). After stirring for 30 minutes at room temperature, compound 5-2 (0.64 mL, 5.15 mmol, 1.2 equivalents) was added to the mixture. The mixture was stirred for 2 hours at room temperature, and TLC analysis of the reaction showed complete conversion to the desired product. The mixture was then diluted with water (10 mL) and extracted with ethyl acetate (2×10 mL). The combined organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The obtained crude product was purified by silica gel chromatography (petroleum ether: ethyl acetate, 84:16) to obtain compound 5-3 (1.23 g, 95%) as a white solid. TLC conditions: petroleum ether: ethyl acetate = 5:1, UV 254nm. The Rf of compound 5-1 was 0.2, and the Rf of compound 5-3 was 0.6.
References:
CN112480100,2021,A Location in patent:Paragraph 0103; 0108-0109; 0135; 0139-0140
![1H-Pyrrolo[2,3-b]pyridine, 1-(phenylsulfonyl)-](/CAS/GIF/143141-23-5.gif)
143141-23-5
78 suppliers
$30.00/1g
![1H-Pyrrolo[2,3-b]pyridine, 5-nitro-1-(phenylsulfonyl)-](/CAS/GIF/937012-11-8.gif)
937012-11-8
21 suppliers
inquiry