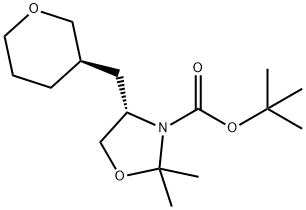
3-Oxazolidinecarboxylic acid, 2,2-diMethyl-4-[[(3R)-tetrahydro-2H-pyran-3-yl]Methyl]-, 1,1-diMethylethyl ester, (4S)- synthesis
- Product Name:3-Oxazolidinecarboxylic acid, 2,2-diMethyl-4-[[(3R)-tetrahydro-2H-pyran-3-yl]Methyl]-, 1,1-diMethylethyl ester, (4S)-
- CAS Number:942144-34-5
- Molecular formula:C16H29NO4
- Molecular Weight:299.41
![3-Oxazolidinecarboxylic acid, 4-[(2R)-2-[[[(1,1-diMethylethyl)diMethylsilyl]oxy]Methyl]-5-[(Methylsulfonyl)oxy]pentyl]-2,2-diMethyl-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-36-7.gif)
942144-36-7
6 suppliers
inquiry
![3-Oxazolidinecarboxylic acid, 2,2-diMethyl-4-[[(3R)-tetrahydro-2H-pyran-3-yl]Methyl]-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-34-5.gif)
942144-34-5
5 suppliers
inquiry
Yield:942144-34-5 54%
Reaction Conditions:
with N,N,N-tributylbutan-1-aminium fluoride;lithium hydroxide monohydrate in tetrahydrofuran;Reflux;
Steps:
1.7
Step 7. (S)-ter(-buty 2,2-dimethyl-4-(((Λ)-tetrahydro-2H-pyran-3-yl)methyl)oxazolidine-3- carboxylate; To a solution of (S)-tert-buty 4-(CK)-2-((tert-butyldimethylsilyloxy)methyl)-5-(methylsulfonyloxy)pentyl)-2,2-dimethyloxazolidine-3-carboxylate (37.7 g, 74.2 mmol) in THF (1000 mL) was added tetraethylammonium fluoride hydrate (41 g, 185.5 mmol) in portions. The reaction mixture was stirred under reflux overnight. The mixture was diluted with EtOAc (1000 mL), washed with water (300 mL) and brine (500 mL). The organic phase was dried over Na2SO4, filtered and concentrated in vacuo to give the crude product, which was purified by column chromatography to afford (S)-tert-buty 2,2-dimethyl-4-(((/?)-tetrahydro-2H-pyran-3- yl)methyl)oxazolidine-3-carboxylate (12.0 g, 54%).
References:
WO2009/154766,2009,A1 Location in patent:Page/Page column 36; 38
![CarbaMic acid, N-[(1S,3R)-1,3-bis(hydroxyMethyl)-5-hexen-1-yl]-, 1,1-diMethylethyl ester](/CAS/GIF/942144-12-9.gif)
942144-12-9
5 suppliers
inquiry
![3-Oxazolidinecarboxylic acid, 2,2-diMethyl-4-[[(3R)-tetrahydro-2H-pyran-3-yl]Methyl]-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-34-5.gif)
942144-34-5
5 suppliers
inquiry
![3-Oxazolidinecarboxylic acid, 4-[(2R)-2-(hydroxyMethyl)-4-penten-1-yl]-2,2-diMethyl-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-13-0.gif)
942144-13-0
6 suppliers
inquiry
![3-Oxazolidinecarboxylic acid, 2,2-diMethyl-4-[[(3R)-tetrahydro-2H-pyran-3-yl]Methyl]-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-34-5.gif)
942144-34-5
5 suppliers
inquiry
153080-81-0
8 suppliers
inquiry
![3-Oxazolidinecarboxylic acid, 2,2-diMethyl-4-[[(3R)-tetrahydro-2H-pyran-3-yl]Methyl]-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-34-5.gif)
942144-34-5
5 suppliers
inquiry
![3-Oxazolidinecarboxylic acid, 4-[(2R)-2-[[[(1,1-diMethylethyl)diMethylsilyl]oxy]Methyl]-4-penten-1-yl]-2,2-diMethyl-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-14-1.gif)
942144-14-1
7 suppliers
inquiry
![3-Oxazolidinecarboxylic acid, 2,2-diMethyl-4-[[(3R)-tetrahydro-2H-pyran-3-yl]Methyl]-, 1,1-diMethylethyl ester, (4S)-](/CAS/GIF/942144-34-5.gif)
942144-34-5
5 suppliers
inquiry