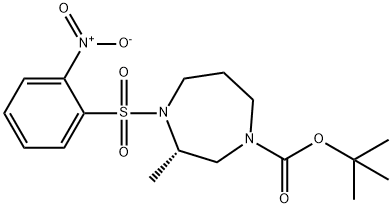
(S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylate synthesis
- Product Name:(S)-tert-Butyl 3-methyl-4-((2-nitrophenyl)sulfonyl)-1,4-diazepane-1-carboxylate
- CAS Number:949109-36-8
- Molecular formula:C17H25N3O6S
- Molecular Weight:399.46
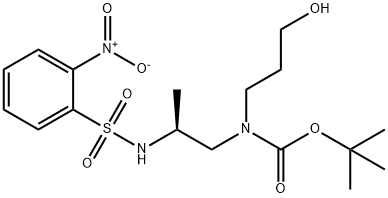
949109-35-7

949109-36-8
(iv) Preparation of tert-butyl (S)-4-(2-nitrophenylsulfonyl)-3-methyl-1,4-diazepane-1-carboxylate: to a solution of (S)-3-hydroxypropyl-2-butyl ester (7.00 kg, 16.8 mol) and triphenylphosphorane (4.90 kg, 18.7 mol) in tetrahydrofuran (60 L) at 5 °C under nitrogen protection, slowly dropwise addition of Diisopropyl azodicarboxylate (4.60 kg, 22.7 mol) over 2 hours. The reaction mixture was stirred at room temperature for 7 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming complete consumption of the raw materials, the reaction mixture was concentrated under reduced pressure. A solvent mixture of petroleum ether and methyl tert-butyl ether (12.5:1, v/v) was added to the concentrated residue and stirred vigorously. The precipitate was removed by filtration and the filtrate was concentrated under reduced pressure. Petroleum ether was again added to the residue, and the precipitate was collected by filtration with vigorous stirring and dried to give a yellow solid target product (5.00 kg, 74.5% yield). The product was characterized by 1H-NMR (DMSO-d6, 80 °C): δ 0.89 (3H, d, J = 6.8 Hz), 1.40 (9H, s), 1.65-1.72 (2H, m), 3.05-3.14 (2H, m), 3.25 (1H, ddd, J = 15.6, 7.0, 7.0 Hz), 3.63 (2H, dd, J = 15.6, 5.4 Hz), 3.73 (1H, ddd, J = 15.6, 4.0, 4.0 Hz), 4.22-4.30 (1H, m), 7.79-7.88 (3H, m), 7.98 (1H, dd, J = 7.6, 2.0 Hz). Melting point: 113-114°C.

949109-35-7
1 suppliers
inquiry

949109-36-8
16 suppliers
inquiry
Yield:949109-36-8 90%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 20;Mitsunobu Displacement;
References:
Gomi, Noriaki;Shibuya, Kimiyuki;Kawamura, Kiyoshi;Kabeya, Mototsugu [Tetrahedron Letters,2022,vol. 91,art. no. 153589]

24424-99-5
868 suppliers
$13.50/25G
![1H-1,4-Diazepine, hexahydro-2-methyl-1-[(2-nitrophenyl)sulfonyl]-, hydrochloride (1:1), (2S)-](/CAS/20211123/GIF/1360538-88-0.gif)
1360538-88-0
0 suppliers
inquiry

949109-36-8
16 suppliers
inquiry
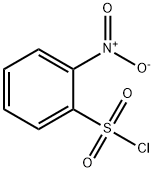
1694-92-4
23 suppliers
$13.31/25G

949109-36-8
16 suppliers
inquiry

1360538-87-9
1 suppliers
inquiry

949109-36-8
16 suppliers
inquiry