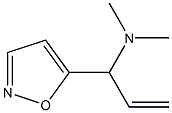
5-Isoxazolemethanamine, .alpha.-ethenyl-N,N-dimethyl- synthesis
- Product Name:5-Isoxazolemethanamine, .alpha.-ethenyl-N,N-dimethyl-
- CAS Number:951698-15-0
- Molecular formula:C15H18N2O2
- Molecular Weight:258.32

951698-14-9
1 suppliers
inquiry

124-40-3
546 suppliers
$18.00/100ml

951698-15-0
27 suppliers
inquiry
Yield: 80%
Reaction Conditions:
Stage #1:C13H13NO3 with methanesulfonyl chloride;triethylamine in dichloromethane at -12 - -10; for 0.5 - 40 h;
Stage #2:dimethyl amine in tetrahydrofuran;dichloromethane at -40 - 15; for 4.5 - 7 h;
Steps:
1; 2.B
Amine JDB6:[0094] Methanesulfonyl chloride (589 μL, 7.46 mmol, 1.15 equiv) was added dropwise via syringe to a solution of alcohol JDB5 (1.50 g, 6.49 mmol, 1 equiv) and triethylamine (1.18 mL, 8.44 mmol, 1.30 equiv) in dichloromethane (65 mL) at -10 0C. After stirring at -10 0C for 40 min, the reaction mixture was cooled to -30 0C and a solution of dimethylamine in tetrahydrofuran (5.30 M, 7.40 mL, 38.9 mmol, 6.00 equiv) was added via syringe. The reaction mixture was allowed to warm slowly to 5 0C over 4.5 h, then was partitioned between aqueous potassium phosphate buffer (pH 7.0, 0.05 M, 50 mL) and dichloromethane (100 mL). The aqueous layer was further extracted with dichloromethane (50 mL). The organic layers were combined and the combined layers were washed with saturated aqueous sodium chloride solution (50 mL). The washed solution was dried over sodium sulfate and the solids were filtered. The filtered solution was concentrated and the residue obtained was purified by flash-column chromatography on silica gel (0.5% methanol- dichloromethane, grading to 3% methanol-dichloromethane) to furnish the allylic amine JDB6 (1.34 g, 80%) as a clear, colorless oil.TLC (40% acetone-hexanes) R/= 0.42 (UV, CAM).HNMR (500 MHz, CDCl3), δ: 7.46-7.36 (m, 5H, ArH), 5.94 (m, IH, CH2=CH), 5.81(s, IH, IsoxH), 5.32-5.29 (m, 2H, CHH=CH), 5.26 (s,2H, OCH2Ar), 2.27 (s, 6H, N(CHa)2).13 CNMR (100 MHz, CDCl3), δ: 172.5, 171.6, 135.8, 134.1, 128.6, 128.5, 128.3, 119.5,93.9, 71.5, 66.3, 42.3.IR (neat), cm" 2946 (w), 2869 (w), 2827 (w), 2782 (w), 1607 (s), 1501(s), 1449 (s), 1366 (s), 1138 (w), 1036 (s), 992 (s), 926(S).HRMS (ESI): Calcd for (Ci5Hi8N2C^H)+: 259.1446Found: 259.1436.; [00105] B. A 12-L, Morton-style, four-necked flask was equipped with a mechanical stirrer and a thermocouple. The system was flushed with argon and charged with a solution of unpurified allylic alcohol 4 (369 mmol, 1 equiv) and triethylamine (67.3 mL, 480 mmol, 1.30 equiv) in dichloromethane (3.7 L). The solution was cooled to -12 0C in a dry ice- acetone bath, and then methanesulfonyl chloride (33.5 mL, 424 mmol, 1.15 equiv) was added dropwise via syringe. After stirring at -12 0C for 30 min, the reaction mixture was cooled to -30 0C by addition of dry ice to the cooling bath and then a solution of dimethylamine in tetrahydrofuran (2.0 M, 1.1 L, 2.2 mol, 6.0 equiv) was added via cannula over 70 min. The reaction mixture was allowed to warm slowly to 15 0C over 7 h, and then was concentrated to a volume of 1.5 L. The reaction mixture was partitioned between aqueous potassium phosphate buffer (pH 7.0, 0.05 M, 2 L) and dichloromethane (1.5 L). The aqueous layer was further extracted with dichloromethane (1 L). The organic layers were combined and the combined layers were dried over sodium sulfate. The solids were filtered and the filtrate was concentrated. The residue obtained was partitioned between diethyl ether (700 mL) and aqueous hydrochloric acid solution (1.0 M, IL). The hydrochloric acid extraction was cooled in an ice- water bath and brought to pH 13 by slow addition of aqueous sodium hydroxide solution (6.0 M). The basic aqueous mixture was extracted with diethyl ether (2 x 700 mL) and the organic layers were combined. The combined organic layers were washed with saturated aqueous sodium chloride solution, dried over sodium sulfate, and the dried solution was filtered. The filtrate was concentrated, and the residue was purified by flash-column chromatography on silica gel (3% methanol-dichloromethane) to furnish the allylic amine 5 (76.3 g, 80% over two steps) as a pale yellow oil.; Tertiary Amine 5: TLC (40% acetone-hexanes) R/= 0.42 (UV, CAM).HNMR (500 MHz, CDCl3), δ: 7.46-7.36 (m, 5H, ArH), 5.94 (m, IH, CH2=CH), 5.81(s, IH, IsoxH), 5.32-5.29 (m, 2H, CHH=CH), 5.26 (s,2H, OCH2Ar), 4.00 (d, IH, J = 7.8 Hz, CHN(CH3)2),2.27 (s, 6H, N(CH3)2).13 CNMR (100 MHz, CDCl3), δ: 172.5, 171.6, 135.8, 134.1, 128.6, 128.5, 128.3, 119.5,93.9, 71.5, 66.3, 42.3. IR (neat), cm" 2946 (w), 2869 (w), 2827 (w), 2782 (w), 1607 (s), 1501 (s), 1449 (s), 1366 (s), 1138 (w), 1036 (s), 992 (s), 926(S).HRMS (ESI): Calcd for (Ci5H18N2O2+H)+: 259.1446 Found: 259.1436.
References:
PRESIDENT AND FELLOWS OF HARVARD COLLEGE WO2008/127361, 2008, A2 Location in patent:Page/Page column 68-69; 77-79
2552-53-6
31 suppliers
inquiry

951698-15-0
27 suppliers
inquiry

951698-16-1
0 suppliers
inquiry

951698-15-0
27 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

951698-15-0
27 suppliers
inquiry

951698-14-9
1 suppliers
inquiry

951698-15-0
27 suppliers
inquiry