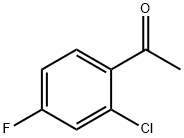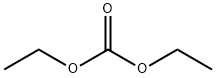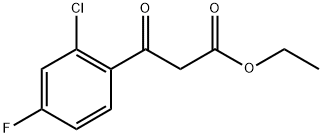
Benzenepropanoic acid, 2-chloro-4-fluoro-β-oxo-, ethyl ester synthesis
- Product Name:Benzenepropanoic acid, 2-chloro-4-fluoro-β-oxo-, ethyl ester
- CAS Number:951784-80-8
- Molecular formula:C11H10ClFO3
- Molecular Weight:244.65
Yield:951784-80-8 40%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil; for 17 h;Reflux;
Steps:
12.1 12.1 Ethyl 3 -(2-chloro-4-fluorophenyl)-3 -oxopropanoate
General procedure: General Method 1: Beta-ketoester Synthesis:_To a solution of an acetophenone (1 equiv) in THF (10 vol) was added a dialkyl carbonate (2 equiv) and NaH (60%, 2 equiv). The solution was heated to reflux for 17 hours, before cooling to 0°C with the aid of an ice bath. The dark brown mixture was carefully acidified to pH 4 with 36% HC1 and filtered to remove inorganic impurities. The filtrate was poured into a large amount of water (50 vol) and extracted with ethyl acetate (3 x 10 vol). The combined extracts were washed with brine (15 vol),dried over magnesium sulfate, filtered, and concentrated under reduced pressure. As required, the product was then purified via a standard method(s).:_Ethyl 3-(2-chloro-4-fluorophenyl)-3-oxopropanoate was synthesized from 1 -(2-chloro-4- fluorophenyl)ethanone and diethyl carbonate according to General Method 1 and was chromatographed by silica gel column chromatography eluting with heptanes:ethyl acetate gradients to provide the titlecompound as an orange oil (3.1 g, 40% yield). LC-MS: tR=l.98 mi [M+H]=244.9.
References:
WO2015/183839,2015,A1 Location in patent:Page/Page column 179; 203
1999-00-4
212 suppliers
$13.00/1g

951784-80-8
6 suppliers
inquiry