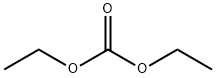
Diethyl carbonate
- CAS No.
- 105-58-8
- Chemical Name:
- Diethyl carbonate
- Synonyms
- ETHYL CARBONATE;EUFIN;CED;Diethylcarbonat;2-CHLORO-N,N-DIETHYLAMINE HYDROCHLORIDE;TGFB;progressive;Diethyl carbote;2-CHLORO-N,N-DIETHYLETHYLAMINE HYDROCHLORIDE;DPD1
- CBNumber:
- CB8852663
- Molecular Formula:
- C5H10O3
- Molecular Weight:
- 118.13
- MDL Number:
- MFCD00009107
- MOL File:
- 105-58-8.mol
- MSDS File:
- SDS
| Melting point | -43 °C (lit.) |
|---|---|
| Boiling point | 126-128 °C (lit.) |
| Density | 0.975 g/mL at 25 °C (lit.) |
| vapor density | 4.1 (vs air) |
| vapor pressure | 10 mm Hg ( 23.8 °C) |
| refractive index |
n |
| Flash point | 88 °F |
| storage temp. | no restrictions. |
| solubility | 18.8mg/l insoluble |
| form | Liquid |
| color | Clear |
| PH | 6.3 (1g/l, H2O) |
| Odor | Pleasant, etheral; mild and nonresidual. |
| explosive limit | 1.4-11.0%(V) |
| Water Solubility | Negligible |
| Sensitive | Moisture Sensitive |
| Merck | 14,3780 |
| BRN | 956591 |
| Dielectric constant | 2.8(Ambient) |
| Stability | Stable. Flammable. Incompatible with strong acids, strong bases, reducing agents, oxidizing agents. Protect from moisture. |
| LogP | 1.21 at 25℃ |
| CAS DataBase Reference | 105-58-8(CAS DataBase Reference) |
| FDA UNII | 3UA92692HG |
| NIST Chemistry Reference | Carbonic acid, diethyl ester(105-58-8) |
| EPA Substance Registry System | Diethyl carbonate (105-58-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P243 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 23/24/25-36/37/38-10 | |||||||||
| Safety Statements | 26-36-45-16-23 | |||||||||
| RIDADR | UN 2811 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YE1050000 | |||||||||
| F | 21 | |||||||||
| Autoignition Temperature | 445 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2920 90 10 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in Rabbit: > 4900 mg/kg LC50 (inhalation) > 21 mg/kg (mouse) TDL0 500 mg/kg (mouse) LD50 (s.c.) 8500 mg/kg (rat) |
|||||||||
| NFPA 704 |
|
Diethyl carbonate price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SAB5300197 | Monoclonal Anti-TGFB1 antibody produced in mouse clone 4F9C10, ascites fluid | 105-58-8 | 100μL | $524 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02898 | Diethyl carbonate for synthesis | 105-58-8 | 100mL | $36.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02898 | Diethyl carbonate for synthesis | 105-58-8 | 500mL | $49.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02898 | Diethyl carbonate for synthesis | 105-58-8 | 1L | $68.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 517135 | Diethyl carbonate anhydrous, ≥99% | 105-58-8 | 100ml | $52.1 | 2024-03-01 | Buy |
Diethyl carbonate Chemical Properties,Uses,Production
Chemical Properties
Diethyl carbonate is esters, beta-enamino esters, carbamates and unsymmetrical alkyl carbonates. It is a colourless liquid with a mild odour. Insoluble in water, soluble in alcohol, ether and other organic solvents.
Uses
Diethyl carbonate (DEC) is generally used in the C-alkoxycarbonylation of enolate anions, aryl and alkyl cyanides. It reacts with 1,2-amino alcohols to yield corresponding 2-oxazolidinones. Additionally, DEC is also used as a component of electrolyte solution in lithium ion batteries.
Production Methods
Diethyl carbonate (DEC), one of the most frequently used aliphatic carbonates, is produced by passing gaseous phosgene in boiling ethanol, in a molar ratio of 1 part of phosgene to 2.5 – 4 parts of ethanol, into a glass or enamel apparatus consisting of a heatable reaction vessel with a reflux condenser and a distillation setup. The reaction gives essentially quantitative yields (≥ 99 %) and also forms hydrogen chloride. Excess ethanol is removed from the reaction mixture by distillation, and DEC is obtained as the residue.
Manufacturing steps: (a) reacting chlorine and carbon monoxide to produce (COCl2); (b) reacting phosgene with ethyl alcohol to make ethyl chlorocarbonate (ClCO2C2H5); (c) reacting ethyl chlorocarbonate with anhydrous ethyl alcohol to produce diethyl carbonate.
Definition
ChEBI: Diethyl carbonate is a carbonate ester.
Application
Diethyl carbonate is a well-known linear organic carbonate that has wide applications. It is an active component of electrolytes used in lithium which is used as a solvent for cellulose ethers, nitro cellulose, manufacture of radio tubes, fixing rare earths to cathode elements, natural and synthetic resin and in erythromycin intramuscular injections. It is also used in the synthesis of 3-ethyl-4-methyl-5-phenyl-3H-oxazol-2-one, phenobarbital, pyrethrum batteries.
Preparation
Diethyl carbonate is prepared by reaction of phosgene and ethyl alcohol to produce ethyl chlorocarbonate followed by reaction with anhydrous ethylalcohol at elevated temperatures. direct synthesis of diethyl carbonate (dec) by carboxylation of ethanol with co2 was investigated over ceria catalysts.
Synthesis Reference(s)
Journal of the American Chemical Society, 104, p. 1769, 1982 DOI: 10.1021/ja00370a068
General Description
Diethyl carbonate appears as a colorless liquid with a mild pleasant odor. It is slightly less dense than water and insoluble in water.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Diethyl carbonate reacts with acids to liberate heat along with ethanol and carbon dioxide. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides.
Health Hazard
High vapor concentrations can cause headache, irritation of eyes and respiratory tract, dizziness, nausea, weakness, loss of consciousness.
Flammability and Explosibility
Flammable
Safety Profile
Wdly toxic by subcutaneous route. Questionable carcinogen with experimental tumorigenic and teratogenic data. A dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also ANHYDRIDES.
Carcinogenicity
A study using male and female mice treated with 0, 50, 250, or 1000 ppm (0–140 mg/kg/day) diethyl carbonate in drinking water indicated no carcinogenic effects.
Purification Methods
Wash the ester (100mL) with an aqueous 10% Na2CO3 (20mL) solution, saturated CaCl2 (20mL), then water (30mL). After drying by standing over solid CaCl2 for 1hour (note that prolonged contact should be avoided because slow combination with CaCl2 occurs), it should be fractionally distilled. Also dry it over MgSO4 and distil it. [Beilstein 3 H 5, 3 I 4, 3 II 4, 3 III 5, 3 IV 5.]
Diethyl carbonate Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5866 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9354 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2966 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
View Lastest Price from Diethyl carbonate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-19 | Diethyl carbonate
105-58-8
|
US $15.00 / KG | 1KG | 99.99% | 50 ton | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-18 | Diethyl carbonate
105-58-8
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2023-07-27 | Diethyl carbonate
105-58-8
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd |
-

- Diethyl carbonate
105-58-8
- US $15.00 / KG
- 99.99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Diethyl carbonate
105-58-8
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Diethyl carbonate
105-58-8
- US $1.50 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
105-58-8(Diethyl carbonate)Related Search:
1of4