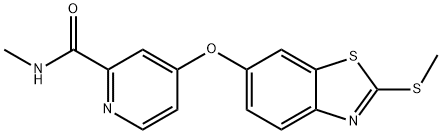
N-Methyl-4-((2-(Methylthio)benzo[d]thiazol-6-yl)oxy)picolinaMide synthesis
- Product Name:N-Methyl-4-((2-(Methylthio)benzo[d]thiazol-6-yl)oxy)picolinaMide
- CAS Number:953770-85-9
- Molecular formula:C15H13N3O2S2
- Molecular Weight:331.41
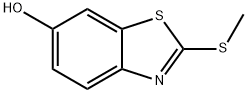
74537-49-8
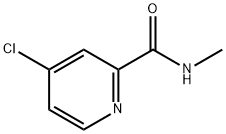
220000-87-3
![N-Methyl-4-((2-(Methylthio)benzo[d]thiazol-6-yl)oxy)picolinaMide](/CAS/20150408/GIF/953770-85-9.gif)
953770-85-9
Step 3. Preparation of N-methyl-4-((2-(methylthio)benzo[d]thiazol-6-yl)oxy)pyridine-2-carboxamide: To a solution of 2-(methylthio)benzo[d]thiazol-6-ol (3.76 g, 19.08 mmol, 1.0 eq.) in DMF (25 mL), Cs2CO3 (15.54 g, 47.70 mmol, 2.5 equivalent) and stirred at room temperature. Subsequently, 4-chloro-N-methylpyridinecarboxamide (4.86 g, 28.62 mmol, 1.5 eq.) was added to the reaction mixture and the mixture was stirred at reflux at 70 °C overnight. After completion of the reaction, the mixture was cooled in an ice bath, water (100 mL) was added and the aqueous layer was extracted with ethyl acetate (3 x 150 mL). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by an ISCO silica gel column (20 g) with an elution gradient of 0%-50%-80%-100% ethyl acetate-hexane mixture at a flow rate of 40 mL/min over 45 min to afford N-methyl-4-((2-(methylthio)benzo[d]thiazol-6-yl)oxy)pyridine-2-carboxamide (3.88 g, 62% yield) as a white solid. Mass spectrometry detected [M+H]+=332.1.

74537-49-8
37 suppliers
$33.00/100mg

220000-87-3
491 suppliers
$8.00/5g
![N-Methyl-4-((2-(Methylthio)benzo[d]thiazol-6-yl)oxy)picolinaMide](/CAS/20150408/GIF/953770-85-9.gif)
953770-85-9
23 suppliers
$57.00/100mg
Yield:953770-85-9 62%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20 - 70;Heating / reflux;
Steps:
15.3 Step 3.
Step 3. Preparation of 4-(2-Methylsulfanyl-benzothiazol-6-yloxy)-pyridine-2-carboxylic acid methylamide To the solution of 2-methylsulfanyl-benzothiazol-6-ol (3.76 g, 19.08 mmol, 1.0 eq) in DMF (25 mL), was added CsCO3 (15.54 g, 47.70 mmol, 2.5 eq) at room temperature. After stirring for a while, 4-chloro-pyridine-2-carboxylic acid methylamide (4.86 g, 28.62 mmol, 1.5 eq) was added to the mixture and the mixture was stirred at 70° C. under reflux condenser overnight. After cooling the reaction mixture in ice bath, water (100 mL) was added and the aqueous layer was extracted with ethyl acetate (3*150 mL). The organic layer was dried over sodium sulfate, filtered, and evaporated in vacuo. The crude product was purified using 20 g of ISCO Silica Gel column (0%-50%-80%-100% ethyl acetate-hexane mixture over 45 min 40 mL/min run) to yield 4-(2-methylsulfanyl-benzothiazol-6-yloxy)-pyridine-2-carboxylic acid methylamide (3.88 g, 62%) as a white solid. M+H=332.1
References:
US2008/45528,2008,A1 Location in patent:Page/Page column 47