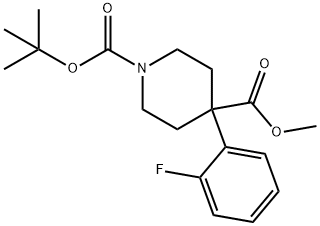
1-BOC-4-(2-FLUOROPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:1-BOC-4-(2-FLUOROPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID METHYL ESTER
- CAS Number:954125-24-7
- Molecular formula:C18H24FNO4
- Molecular Weight:337.39
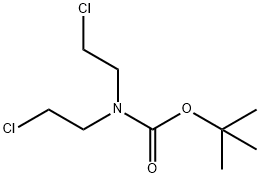
118753-70-1
174 suppliers
$6.00/1g
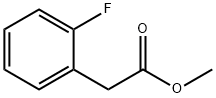
57486-67-6
99 suppliers
$31.00/5g

954125-24-7
3 suppliers
inquiry
Yield:954125-24-7 17%
Reaction Conditions:
Stage #1: methyl o-fluorophenylacetatewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.416667 h;
Stage #2: N-(tert-butyloxycarbonyl)bis(2-chloroethyl)amine in N,N-dimethyl-formamide at 20;
Steps:
1-tert-butyl 4-methyl 4-(2-fluorophenyl)piperidine-1,4-dicarboxylate. A flask was charged with sodium hydride (2.14 g, 89.2 mmol) and dimethylformamide (70 ml) at 0° C. under nitrogen. Methyl 2-(2-fluorophenyl)acetate (5 g, 29.7 mmol) was added to the flask, and after stirring for 25 min, tert-butyl bis(2-chloroethyl)carbamate (8.6 g, 35.6 mmol) was added. The reaction was allowed to warm to rt and stirred overnight. The reaction was quenched with a saturated solution of ammonium chloride and extracted with ethyl acetate (5×100 mL). The organics were collected, washed with brine, dried over sodium sulfate, and concentrated in vacuo. The residue was purified via silica gel chromatography (10/90→50/50 ethyl acetate/hexanes) to yield 1.7 g (17%) of the desired product. 1H-NMR (CDCl3, 400 MHz) δ ppm 7.29 (m, 1H), 7.24 (m, 1H), 7.12 (m, 1H), 7.00 (m, 1H), 3.86 (m, 2H), 3.67 (m, 3H), 3.22 (m, 2H), 2.37 (m, 2H), 1.96 (m, 2H), 1.44 (s, 9H). Mass spec.: 360.22 (MNa)+. LC tr=3.503 min (Phenomenex-Luna 4.6×50 mm S10, 10% MeOH/90% H2O/0.1% TFA→90% MeOH/10% H2O/0.1% TFA Gradient Time=4 min, Flow rate=4 mL/min)
References:
US2007/249607,2007,A1 Location in patent:Page/Page column 93