(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine synthesis
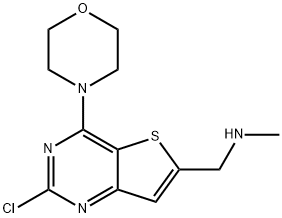
- Product Name:(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine
- CAS Number:955979-15-4
- Molecular formula:C12H15ClN4OS
- Molecular Weight:298.79
![2-CHLORO-4-MORPHOLINOTHIENO[3,2-D]PYRIMIDINE-6-CARBALDEHYDE](/CAS/GIF/885618-31-5.gif)
885618-31-5
![(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine](/CAS/20180703/GIF/955979-15-4.gif)
955979-15-4
Under nitrogen protection, 2-chloro-4-(4-morpholinato)-thieno[3,2-d]pyrimidine-6-carboxaldehyde (20.0 g, 70.4 mmol, 1.0 eq.) was dissolved in methanol (125 mL) followed by addition of methylamine solution (27% v/v, 75 mL, 563.2 mmol). The reaction mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure to give the crude product. The crude product was dissolved in methanol (550 mL) and tetrahydrofuran (220 mL) under nitrogen protection, and sodium borohydride (8 g, 211.2 mmol) was added in batches. The reaction mixture was stirred overnight at room temperature and concentrated under reduced pressure. Water (300 mL) was added to the concentrate and the aqueous phase was extracted with dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate and concentrated. The residue was dissolved in 6 M hydrochloric acid (230 mL) and stirred for 30 minutes. The aqueous phase was washed several times with dichloromethane and the pH was subsequently adjusted to 9-10 with 4N sodium hydroxide solution. the precipitated solid was collected by filtration and dried at 60 °C for 6 h to give a light yellow solid product (18 g, 85% yield). Melting point: 240-245°C. LCMS (m/z): 299 [M + 1]+. 1H NMR (400MHz, DMSO-d6): δ 2.32 (s, 3H), 3.74 (t, J=5.2Hz, 4H), 3.88 (t, J=5.2Hz, 4H), 3.96 (s, 2H), 7.24 (s, 1H).
![2-CHLORO-4-MORPHOLINOTHIENO[3,2-D]PYRIMIDINE-6-CARBALDEHYDE](/CAS/GIF/885618-31-5.gif)
885618-31-5
69 suppliers
$69.00/100mg

74-89-5
0 suppliers
$13.44/25ML
![(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine](/CAS/20180703/GIF/955979-15-4.gif)
955979-15-4
17 suppliers
inquiry
Yield:955979-15-4 90.9%
Reaction Conditions:
Stage #1: 2-chloro-4-morpholinothieno[3,2-d]pyrimidine-6-carbaldehyde;methylamine in methanol at 20 - 30;Large scale;
Stage #2: with sodium tetrahydroborate at 20 - 30;Large scale;
Steps:
1.1-g (7) Step 1-g: Preparation of N- (2-chloro-4-morpholinothiophene [3,2-d] pyrimidine-6methyl) -methanamine (Compound 106)Prepared.
A solution of 30% methanol (60.5 kg, 584.35 mol, 8.0 equiv) was added to compound 105 (20.6 thousandG, 72.7 mol, 1 eq.) In methanol (115 kg). The reaction mixture is stirred at 20 to 30 degrees Celsius for 10 to 12 hours. The mixture was concentrated under reduced pressure. Methanol (405 kg) and tetrahydrofuran (185 kg)Was added to the concentrated mixture, and then anhydrous magnesium sulfate (20.6 kg) was added to ensure that the reaction system was dry throughout the reaction.Sodium borohydride (10 kg, 264.34 mol, 3.64 equiv) was added portionwise to the mixture,The mixture was stirred at 20 to 30 degrees Celsius for 6 to 7 hours. Water (110 kg) was added to the mixture at the end of the reaction and stirred at 20 to 30 ° C for 30 minutes. The mixture was concentrated under reduced pressure in vacuo. After concentration, 6M hydrochloric acid solution (222 kg) and dichloromethane (181 kg) were added. The reaction mixture is stirred at 20 to 30 degrees Celsius for 30 to 40 minutes, allowed to stand and the organic phase is separated. The water phase is 4MSodium hydroxide solution (316.0 kg) to adjust the pH to 9 ~ 10. The mixture was stirred at 20 to 30 degrees Celsius for 1 to 2 hours. The precipitated solid was centrifuged and dried at 40-50 ° C for 60 hours to give N- (2-chloro-4-morpholinothiazine [3,2-d] pyrimidine-6methyl) -methyl Amine (29.5 kg, yield: 90.9%).
References:
CN104292242,2017,B Location in patent:Paragraph 0129; 0130; 0131
![2-CHLORO-4-MORPHOLINOTHIENO[3,2-D]PYRIMIDINE-6-CARBALDEHYDE](/CAS/GIF/885618-31-5.gif)
885618-31-5
69 suppliers
$69.00/100mg
![(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine](/CAS/20180703/GIF/955979-15-4.gif)
955979-15-4
17 suppliers
inquiry
![6-BroMoMethyl-2-chloro-4-(Morpholin-4-yl)-thieno[3,2-d]pyriMidine](/CAS/GIF/885698-98-6.gif)
885698-98-6
10 suppliers
$504.19/5MG

74-89-5
0 suppliers
$13.44/25ML
![(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine](/CAS/20180703/GIF/955979-15-4.gif)
955979-15-4
17 suppliers
inquiry
![2,4-Dichlorothieno[3,2-d]pyrimidine](/CAS/GIF/16234-14-3.gif)
16234-14-3
277 suppliers
$3.00/1g
![(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine](/CAS/20180703/GIF/955979-15-4.gif)
955979-15-4
17 suppliers
inquiry
![2-Chloro-4-(morpholin-4-yl)thieno[3,2-d]pyrimidine](/CAS/GIF/16234-15-4.gif)
16234-15-4
104 suppliers
$25.00/100mg
![(2-chloro-4-morpholin-4-yl-thieno[3,2-d]pyrimidin-6-ylmethyl)-methyl-amine](/CAS/20180703/GIF/955979-15-4.gif)
955979-15-4
17 suppliers
inquiry