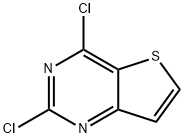
2,4-Dichlorothieno[3,2-d]pyrimidine synthesis
- Product Name:2,4-Dichlorothieno[3,2-d]pyrimidine
- CAS Number:16234-14-3
- Molecular formula:C6H2Cl2N2S
- Molecular Weight:205.06
![1,3-Dihydrothiopheno[3,2-d]pyrimidine-2,4-dione](/CAS/GIF/16233-51-5.gif)
16233-51-5
![2,4-Dichlorothieno[3,2-d]pyrimidine](/CAS/GIF/16234-14-3.gif)
16234-14-3
General procedure for the synthesis of 2,4-dichlorothieno[3,2-d]pyrimidine from thieno[3,2-d]pyrimidine-2,4-dione: To a suspension of thieno[3,2-d]pyrimidine-2,4-dione (5 g, 29 mmol) in POCl3 (40 mL) was added diisopropylethylamine (13 mL, 74 mmol), and the reaction mixture was heated refluxed for 2 hours. Upon completion of the reaction, the excess POCl3 and diisopropylethylamine were removed by distillation under reduced pressure. The resulting brown solid was dissolved in chloroform followed by aqueous phase partitioning. The aqueous phase was adjusted to alkaline by adding 5 M NaOH solution and extracted twice with chloroform. The organic phases were combined, washed sequentially with water and brine, dried over anhydrous Na2SO4 and filtered, and concentrated to afford the target product 2,4-dichlorothieno[3,2-d]pyrimidine as a light brown solid (6.05 g, quantitative yield).1H NMR (CDCl3, 300 MHz): δ 8.16 (d, J = 5.4 Hz, 1H), 7.56 (d, J = 5.7 Hz, 1H); LRMS (ESI): m/z calculated values [M + H]+ 204.94, 206.94, measured values 205.1, 207.0.
![1,3-Dihydrothiopheno[3,2-d]pyrimidine-2,4-dione](/CAS/GIF/16233-51-5.gif)
16233-51-5
166 suppliers
$6.00/250mg
![2,4-Dichlorothieno[3,2-d]pyrimidine](/CAS/GIF/16234-14-3.gif)
16234-14-3
277 suppliers
$3.00/1g
Yield:16234-14-3 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate for 2 h;Heating / reflux;
Steps:
To a suspension of the uracil (5g, 29mmol) in POCI3 (4OmL) was added diisopropylethylamine (13mL, 74mmol) and the reaction heated at reflux for 2h. The excess POCb and diisopropylethylamine were then removed by distillation under reduced pressure and the brown solid obtained dissolved in chloroform and partitioned against water. The aqueous phase was made basic by the addition of 5M NaOH, and extracted twice further with chloroform. The combined organic fractions were washed with water and brine, dried (Na2SO4) filtered and concentrated to afford the product as a pale brown solid (6.05g, quantitative yield). 1H NMR (CDCl3, 300 MHz): 8.16 (d, J=5.4Hz, IH), 7.56 (d, J=5.7Hz, IH); LRMS (ESI): m/z calcd for [M+H]+ 204.94, 206.94 found 205.1, 207.0.
References:
WO2009/62258,2009,A1 Location in patent:Page/Page column 91
![1,3-Dihydrothiopheno[3,2-d]pyrimidine-2,4-dione](/CAS/GIF/16233-51-5.gif)
16233-51-5
166 suppliers
$6.00/250mg
![2,4-Dichlorothieno[3,2-d]pyrimidine](/CAS/GIF/16234-14-3.gif)
16234-14-3
277 suppliers
$3.00/1g
![1,3-Dihydrothiopheno[3,2-d]pyrimidine-2,4-dione](/CAS/GIF/16233-51-5.gif)
16233-51-5
166 suppliers
$6.00/250mg
![2,4-Dichlorothieno[3,2-d]pyrimidine](/CAS/GIF/16234-14-3.gif)
16234-14-3
277 suppliers
$3.00/1g
22288-78-4
394 suppliers
$11.00/5g
![2,4-Dichlorothieno[3,2-d]pyrimidine](/CAS/GIF/16234-14-3.gif)
16234-14-3
277 suppliers
$3.00/1g